Oxidative Transformation of Ethylbenzene Utilizing Metal Bound Immobilized Catalysts
DOI:
https://doi.org/10.31489/2959-0663/4-25-2Keywords:
immobilization, catalyst, nickel, copper, recycle, oxidation, ethylbenzene, polymerAbstract
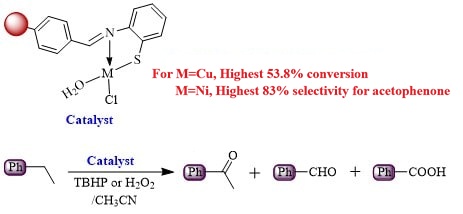
In the present contribution, we have investigated the catalytic potential of immobilized metal catalysts for the oxidation of ethylbenzene. Chloromethylated polystyrene was initially converted to aldehydopolystyrene followed by its functionalization with 2-aminothiophenol to get functionalized resin. This resin was successfully binded with appropriate metal (Mn(II), Fe(III), Ni(II), Cu(II), VO(II)) salts to prepare different heterogeneous catalysts which were characterized using CHNS, energy dispersive X-ray spectroscopy (EDX), diffuse reflectance spectra (DRS), fourier transform infrared spectra (FTIR), atomic absorption spectrophotometry (AAS) and electron paramagnetic resonance (EPR) spectra techniques. Metal binding in mmol per gram of resin for different catalysts was found in the range 0.96–1.24. The catalytic potential of these supported catalysts was assessed for the oxidation of ethylbenzene, utilizing hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (TBHP) as the oxidants. The effects of various reaction parameters, such as temperature, reaction duration, type of oxidant, and the amount of catalyst used were examined for the oxidation reaction. Under optimized milder conditions, the results showed that H2O2 as oxidants resulted in 53.8 % conversion rates with the copper catalyst. The nickel catalyst, when used with H2O2, showed the highest selectivity for acetophenone, reaching 83 %. The recovered catalysts retained its original structure, as established by a comparative analysis of DRS and FT-IR spectra of both the original and reused catalysts. Moreover, the catalysts can be employed repeatedly up to five times under optimum conditions without any noticeable reduction in activity.
References
Nørskov, J. K., Studt, F., Abild-Pedersen, F., & Bligaard, T. (2014). Fundamental concepts in heterogeneous catalysis. John Wiley & Sons. https://doi.org/10.1002/9781118892114 DOI: https://doi.org/10.1002/9781118892114
Spargo, P. L. (2005). Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals. Second Revised and Enlarged Edition. (2-Volume Set.) Edited by Matthias Beller and Carsten Bolm. Wiley-VCH: Weinheim. 2004. 662 pp. (Vol. 1), 652 pp. (Vol. 2). £270. ISBN 3-527-30613-7. Organic Process Research & Development, 9(6), 1017–1018. https://doi.org/10.1021/op050145w DOI: https://doi.org/10.1021/op050145w
Ayogu, J. I., & Onoabedje, E. A. (2019). Recent advances in transition metal-catalysed cross-coupling of (hetero)aryl halides and analogues under ligand-free conditions. Catalysis Science & Technology, 9(19), 5233-5255. https://doi.org/10.1039/C9CY01331H DOI: https://doi.org/10.1039/C9CY01331H
Maharana, T., Nath, N., Pradhan, H. C., Mantri, S., Routaray, A., & Sutar, A. K. (2022). Polymer-supported first-row transition metal schiff base complexes: Efficient catalysts for epoxidation of alkenes. Reactive and Functional Polymers, 171, 105142. https://doi.org/10.1016/j.reactfunctpolym.2021.105142 DOI: https://doi.org/10.1016/j.reactfunctpolym.2021.105142
Sharma, A. S., Sharma, V. S., Yadav, P., Kaur, H., & Varma, R. S. (2023). Polystyrene Resins: Versatile and Economical Support for Heterogeneous Nanocatalysts in Sustainable Organic Reactions. ChemCatChem, 15(8), e202201493. https://doi.org/10.1002/cctc.202201493
Wang, K., Horlyck, J., An, N., & Voutchkova-Kostal, A. (2024). Homogeneous vs. heterogeneous catalysts for acceptorless dehydrogenation of biomass-derived glycerol and ethanol towards circular chemistry. Green Chemistry, 26(7), 3546–3564. https://doi.org/10.1039/D3GC04378A DOI: https://doi.org/10.1039/D3GC04378A
Kumari, S., Kumar, S., Karan, R., Bhatia, R., Kumar, A., Rawal, R. K., & Gupta, P. K. (2024). Synthetic and catalytic perspectives of polystyrene supported metal catalyst. Journal of the Iranian Chemical Society, 21(4), 951–1010. https://doi.org/10.1007/s13738-024-02970-7 DOI: https://doi.org/10.1007/s13738-024-02970-7
Corain, B., Zecca, M., Canton, P., &Centomo, P. (2010). Synthesis and catalytic activity of metal nanoclusters inside functional resins: an endeavour lasting 15 years. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 368(1915), 1495–1507. https://doi.org/10.1098/rsta.2009.0278 DOI: https://doi.org/10.1098/rsta.2009.0278
Sharma, A. S., Sharma, V. S., Yadav, P., Kaur, H., & Varma, R. S. (2023). Polystyrene Resins: Versatile and Economical Support for Heterogeneous Nanocatalysts in Sustainable Organic Reactions. ChemCatChem, 15(8), e202201493. https://doi.org/10.1002/cctc.202201493 DOI: https://doi.org/10.1002/cctc.202201493
Králik, M., & Biffis, A. (2001). Catalysis by metal nanoparticles supported on functional organic polymers. Journal of Molecular Catalysis A: Chemical, 177(1), 113–138. https://doi.org/10.1016/s1381-1169(01)00313-2 DOI: https://doi.org/10.1016/S1381-1169(01)00313-2
Azeez, M. O., Nafiu, S. A., Olarewaju, T. A., Olabintan, A. B., Tanimu, A., Gambo, Y., &Aitani, A. (2023). Selective catalytic oxidation of ethylbenzene to acetophenone: a review of catalyst systems and reaction mechanisms. Industrial & Engineering Chemistry Research, 62(33), 12795–12828. https://doi.org/10.1021/acs.iecr.3c01588
Siddiqi, I., & Pitre, K. S. (2003). Determination of phenones in a perfume composition. Reviews in Analytical Chemistry, 22(1), 9–18. https://doi.org/10.1515/REVAC.2003.22.1.9 DOI: https://doi.org/10.1515/REVAC.2003.22.1.9
Kizilcan, N., Galioğlu, O., & Akar, A. (1993). Modified cyclohexanone – formaldehyde and acetophenone – formaldehyde resins. Journal of applied polymer science, 50(4), 577–584. https://doi.org/10.1002/app.1993.070500402 DOI: https://doi.org/10.1002/app.1993.070500402
Zubkov, F. I., & Kouznetsov, V. V. (2023). Traveling across life sciences with acetophenone — a simple ketone that has special multipurpose missions. Molecules, 28(1), 370. https://doi.org/10.3390/molecules28010370 DOI: https://doi.org/10.3390/molecules28010370
Soucy, N. V. (2014). Acetophenone. Encyclopedia of Toxicology, 43–45. https://doi.org/10.1016/b978-0-12-386454-3.01157-x DOI: https://doi.org/10.1016/B978-0-12-386454-3.01157-X
Mohammadi Ziarani, G., Kheilkordi, Z., & Mohajer, F. (2020). Recent advances in the application of acetophenone in heterocyclic compounds synthesis. Journal of the Iranian Chemical Society, 17, 247–282. https://doi.org/10.1007/s13738-019-01774-4 DOI: https://doi.org/10.1007/s13738-019-01774-4
Loch, C., Reusch, H., Ruge, I., Godelmann, R., Pflaum, T., Kuballa, T., & Lachenmeier, D. W. (2016). Benzaldehyde in cherry flavour as a precursor of benzene formation in beverages. Food Chemistry, 206, 74-77. https://doi.org/10.1016/j.foodchem.2016.03.034 DOI: https://doi.org/10.1016/j.foodchem.2016.03.034
Opgrande, J. L., Dobratz, C. J., Brown, E., Liang, J., Conn, G. S., Shelton, F. J., & With, J. (2000). Benzaldehyde. Kirk‐Othmer Encyclopedia of Chemical Technology. https://doi.org/10.1002/0471238961.0205142615160718.a01 DOI: https://doi.org/10.1002/0471238961.0205142615160718.a01
Mohammadi, A., Mohammadi, S., Bayandori Moghaddam, A., Masoumi, V., & Walker, R. B. (2014). Electropolymerized fluorinated aniline-based fiber for headspace solid-phase microextraction and gas chromatographic determination of benzaldehyde in injectable pharmaceutical formulations. Journal of chromatographic science, 52(9), 971–976. https://doi.org/10.1093/ chromsci/bmt152 DOI: https://doi.org/10.1093/chromsci/bmt152
Theodoropoulou, M. A., Nikitas, N. F., & Kokotos, C. G. (2020). Aldehydes as powerful initiators for photochemical transformations. Beilstein Journal of Organic Chemistry, 16(1), 833–857. https://doi.org/10.3762/bjoc.16.76 DOI: https://doi.org/10.3762/bjoc.16.76
Sieber, R., Bütikofer, U., & Bosset, J. O. (1995). Benzoic acid as a natural compound in cultured dairy products and cheese. International Dairy Journal, 5(3), 227–246. https://doi.org/10.1016/0958-6946(94)00005-A DOI: https://doi.org/10.1016/0958-6946(94)00005-A
Londry, K. L., & Fedorak, P. M. (1992). Benzoic acid intermediates in the anaerobic biodegradation of phenols. Canadian Journal of Microbiology, 38(1), 1–11. https://doi.org/10.1139/m92-001 DOI: https://doi.org/10.1139/m92-001
Faraji, A. R., Mosazadeh, S., & Ashouri, F. (2017). Synthesis and characterization of cobalt-supported catalysts on modified magnetic nanoparticle: green and highly efficient heterogeneous nanocatalyst for selective oxidation of ethylbenzene, cyclohexene and oximes with molecular oxygen. Journal of Colloid and Interface Science, 506, 10–26. https://doi.org/10.1016/J.JCIS. 2017.06.100 DOI: https://doi.org/10.1016/j.jcis.2017.06.100
Gao, L., Zhuge, W., Feng, X., Sun, W., Sun, X., & Zheng, G. (2019). Co/rgo synthesized via the alcohol-thermal method as a heterogeneous catalyst for the highly efficient oxidation of ethylbenzene with oxygen. New Journal of Chemistry, 43(21), 8189–8194. https://doi.org/10.1039/C9NJ00470J DOI: https://doi.org/10.1039/C9NJ00470J
Li, J., Zhao, S., Yang, S., Wang, S., Sun, H., Jiang, S. P., Johannessen, B., & Liu, S. (2021). Atomically dispersed cobalt on graphitic carbon nitride as a robust catalyst for selective oxidation of ethylbenzene by peroxymonosulfate. Journal of Materials Chemistry A, 9, 3029–3035. https://doi.org/10.1039/d0ta11503g DOI: https://doi.org/10.1039/D0TA11503G
Yamazaki, S. (1999). Chromium (VI) oxide-catalyzed benzylic oxidation with periodic acid. Organic Letters, 13(1), 2129–2132. https://doi.org/10.1021/ol991175k DOI: https://doi.org/10.1021/ol991175k
Yang, Y., Zhong, W., Nie, B., Chen, J., Wei, Z., & Liu, X. (2017) Synergetic oxidation of ethylbenzene to acetophenone catalyzed by manganese(II) complexes bearing pendant iodophenyl groups. Journal of Organometallic Chemistry, 853, 136–142. https://doi.org/10.1016/J.JORGANCHEM.2017.10.034 DOI: https://doi.org/10.1016/j.jorganchem.2017.10.034
Venkatesh, G., Vennila, P., Kaya, S., Ahmed, S. B., Sumathi, P., Siva, V., & Kamal, C. (2024). Synthesis and spectroscopic characterization of Schiff base metal complexes, biological activity, and molecular docking studies. ACS omega, 9(7), 8123–8138. https://doi.org/10.1021/acsomega.3c08526 DOI: https://doi.org/10.1021/acsomega.3c08526
Raman, N., Kulandaisamy, A., & Jeyasubramanian, K. (2001). Synthesis, spectroscopic characterization, redox, and biological screening studies of some Schiff base transition metal (II) complexes derived from salicylidene-4-aminoantipyrine and 2 aminophenol/2-aminothiophenol. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 31(7), 1249-1270. https://doi.org/10.1081/SIM-100106862 DOI: https://doi.org/10.1081/SIM-100106862
Islam, M. T., Bitu, M. N. A., Ali, M. A., Hossen, M. F., & Kudrat, M. (2024). Oxovanadium (IV) complexes of α-Amino acid schiff bases and 2,2′-Bipyridine ligands: Synthesis, characterization and investigation of their biological potency. http://dx.doi.org/10.52711/0974-4150.2024.00020 DOI: https://doi.org/10.52711/0974-4150.2024.00020
Aly, A. A., Ghandour, M., & Alfakeh, M. S. (2012). Synthesis and characterization of transition metal coordination polymers derived from 1,4-benzenedicarboxylate and certain azoles. Turkish Journal of Chemistry, 36(1), 69–79. https://doi.org/10.3906/kim-1106-61 DOI: https://doi.org/10.3906/kim-1106-61
Al-Fakeh, M. S. (2020). Synthesis, thermal stability and kinetic studies of copper (II) and cobalt (II) complexes derived from 4-aminobenzohydrazide and 2-mercaptobenzothiazole. European Chemical Bulletin, 9(12), 403–409. http://dx.doi.org/10.17628/ecb.2020.9.403-409 DOI: https://doi.org/10.17628/ecb.2020.9.403-409
Maurya, M. R., Chauhan, A., Arora, S., & Gupta, P. (2022). Triazole based oxidovanadium (V) complex supported on chloromethylated polymer and its catalytic activity for the synthesis of dihydropyrimidinones (DHPMs). Catalysis Today, 397, 3–15. https://doi.org/10.1016/j.cattod.2022.03.006 DOI: https://doi.org/10.1016/j.cattod.2022.03.006
Gomathi, V., & Selvameena, R. (2022). Synthesis, structural analysis and antimicrobial screening of Mn (II) complexes of Schiff bases. Journal of the Mexican Chemical Society, 66(1), 70–78. https://doi.org/10.29356/jmcs.v66i1.1621 DOI: https://doi.org/10.29356/jmcs.v66i1.1621
Buldurun, K., Turan, N., Savci, A., Alan, Y., & Colak, N. (2022). Synthesis, characterization, X-ray diffraction analysis of a tridentate Schiff base ligand and its complexes with Co (II), Fe (II), Pd (II) and Ru (II): Bioactivity studies. Iran. J. Chem. Chem. Eng. Research Article Vol, 41(8). https://doi.org/10.30492/ijcce.2021.531629.4775
Dinku, D., Demissie, T. B., Beas, I. N., Eswaramoorthy, R., Abdi, B., & Desalegn, T. (2024). Antimicrobial activities and docking studies of new Schiff base ligand and its Cu (II), Zn (II) and Ni (II) Complexes: Synthesis and Characterization. Inorganic Chemistry Communications, 160, 111903. https://doi.org/10.1016/j.inoche.2023.111903 DOI: https://doi.org/10.1016/j.inoche.2023.111903
Chellaian, J. D., & SS, S. R. (2021). Co (II), Ni (II), Cu (II), and Zn (II) complexes of 4‐aminoantipyrine‐derived Schiff base. Synthesis, structural elucidation, thermal, biological studies, and photocatalytic activity. Journal of Heterocyclic Chemistry, 58(4), 928–941. https://doi.org/10.1002/jhet.4209 DOI: https://doi.org/10.1002/jhet.4209
Vijayan, J. G. (2022). Synthesis, Characterization, Magnetic, Thermal and Electrochemical Studies of Oxovanadium (IV) Complex of 2-thiophenecarba Benzhydrazone. In Advanced Polymeric Materials (pp. 59-72). River Publishers. https://doi.org/10.1201/9781003337041-3 DOI: https://doi.org/10.1201/9781003337041-3
Mureseanu, M., Filip, M., Bleotu, I., Spinu, C. I., Marin, A. H., Matei, I., &Parvulescu, V. (2023). Cu (II) and Mn (II) Anchored on Functionalized Mesoporous Silica with Schiff Bases: Effects of Supports and Metal–Ligand Interactions on Catalytic Activity. Nanomaterials, 13(12), 1884. https://doi.org/10.3390/nano13121884 DOI: https://doi.org/10.3390/nano13121884
Howsaui, H. B., Sharfalddin, A. A., Abdellattif, M. H., Basaleh, A. S., & Hussien, M. A. (2021). Synthesis, spectroscopic characterization and biological studies of Mn (II), Cu (Ii), Ni (II), Co (II) and Zn (II) complexes with new schiff base of 2-((pyrazine-2-ylimino)methyl) phenol. Applied Sciences, 11(19), 9067. https://doi.org/10.3390/app11199067 DOI: https://doi.org/10.3390/app11199067
Azeez, M. O., Nafiu, S. A., Olarewaju, T. A., Olabintan, A. B., Tanimu, A., Gambo, Y., &Aitani, A. (2023). Selective catalytic oxidation of ethylbenzene to acetophenone: a review of catalyst systems and reaction mechanisms. Industrial & Engineering Chemistry Research, 62(33), 12795–12828. https://doi.org/10.1021/acs.iecr.3c01588 DOI: https://doi.org/10.1021/acs.iecr.3c01588
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Savita Kumari, Praveen K. Gupta, Amit Kumar, Ramesh Kumar

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



