Cyclopentane as an Eco-Friendly Alternative: A Review of its Properties, Industrial Applications, and Production Methods
DOI:
https://doi.org/10.31489/2959-0663/4-25-9Keywords:
cyclopentane, fraction C5, pyrolysis, cyclopentene, cyclopentadiene, hydrogenation, cyclopentane hydrates, eco-friendly, c-pentaneAbstract
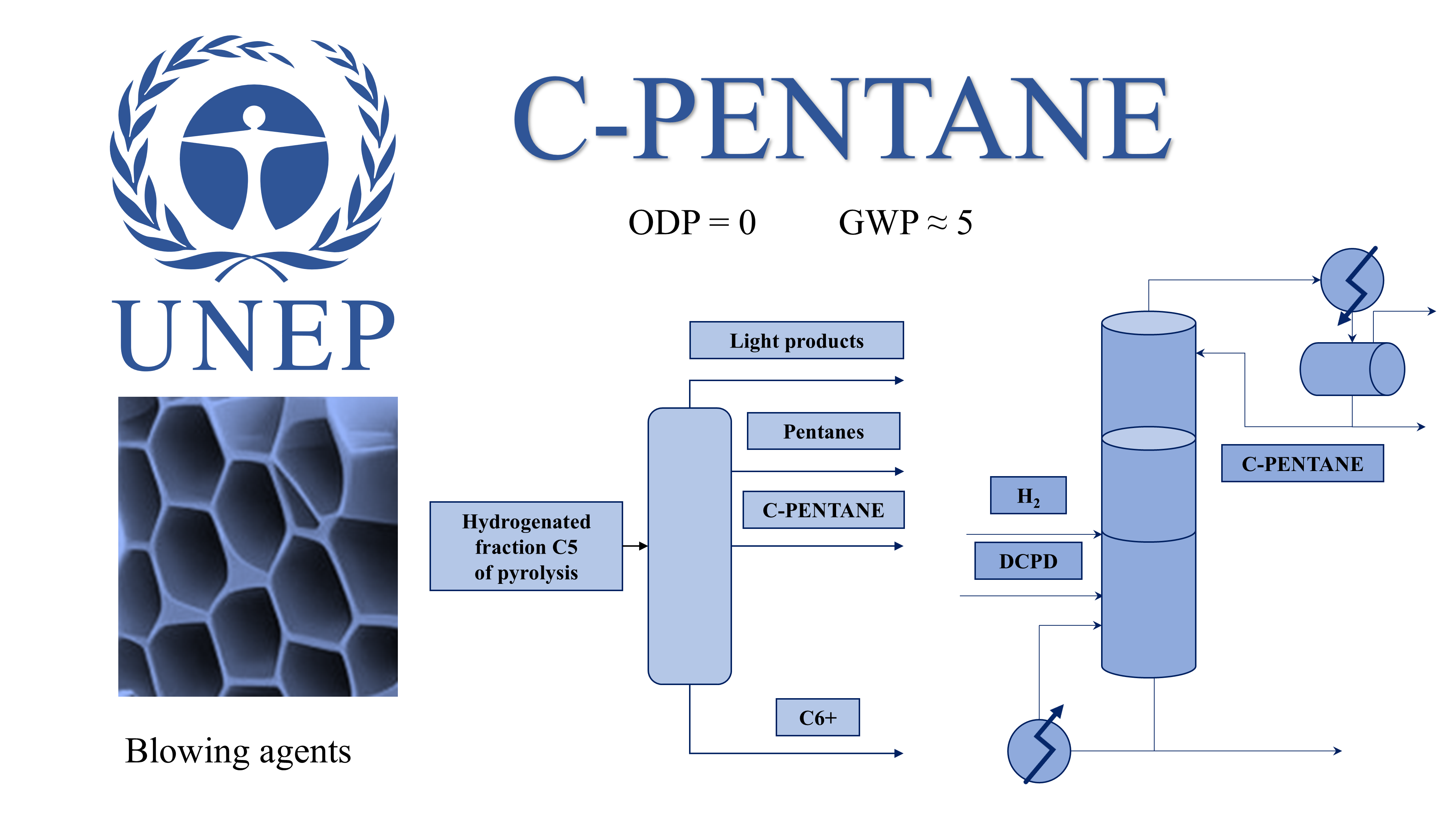
The Montreal Protocol established stringent international regulations concerning the production, consumption, and trade of ozone-depleting substances, including chlorofluorocarbons and hydrofluorocarbons, aimed at safeguarding the Earth’s ozone layer. In this context, cyclopentane has emerged as an environmentally sustainable alternative owing to its zero-ozone depletion potential and low global warming potential. This review examines the physicochemical properties, industrial applications, and production methods of cyclopentane, with particular emphasis on its utilization as a refrigerant, a blowing agent in rigid polyurethane foams, and a hydrate-forming agent for seawater desalination. The primary applications are concentrated in refrigeration and thermal insulation, where cyclopentane-based foams demonstrate superior thermal conductivity and mechanical stability relative to conventional agents. However, the flammability of cyclopentane vapor presents operational challenges that necessitate the implementation of appropriate safety measures. Advances in catalytic reaction-distillation and extractive distillation processes may improve the efficiency of cyclopentane production and product purity in industrial settings. This review underscores cyclopentane’s efficacy as a substitute for compounds with higher ozone depletion potentials and emphasizes the importance of ongoing research into scalable, economically viable production technologies and safe industrial integration to fully realize its environmental and practical benefits.
References
United Nations Environment Programme. Ozone Secretariat. (2006). Handbook for the Montreal protocol on substances that deplete the ozone layer. UNEP/Earthprint. https://ozone.unep.org/sites/default/files/2019-09/MP-Handbook-07-en-2006.pdf
Lide, D. R. (2020). Basic laboratory and industrial chemicals: a CRC quick reference handbook. CRC press. https://doi.org/10.1201/9780429333026 DOI: https://doi.org/10.1201/9780429333026
Pitzer, K. S., & Donath, W. E. (1959). Conformations and strain energy of cyclopentane and its derivatives. Journal of the American Chemical Society, 81(13), 3213–3218. https://doi.org/10.1021/ja01522a014 DOI: https://doi.org/10.1021/ja01522a014
Ouellette, R. J., & Rawn, J. D. (2018). Organic chemistry: structure, mechanism, synthesis. Academic Press. https://doi.org/10.1016/B978-0-12-812838-1.50001-3 DOI: https://doi.org/10.1016/B978-0-12-812838-1.50001-3
Span, R. (2000). Multiparameter equations of state. Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-04092-8 DOI: https://doi.org/10.1007/978-3-662-04092-8_5
Mokbel, I., Rauzy, E., Loiseleur, H., Berro, C., & Jose, J. (1995). Vapor pressures of 12 alkylcyclohexanes, cyclopentane, butylcyclopentane and trans-decahydronaphthalene down to 0.5 Pa. Experimental results, correlation and prediction by an equation of state. Fluid phase equilibria, 108(1-2), 103–120. https://doi.org/10.1016/0378-3812(95)02707-L DOI: https://doi.org/10.1016/0378-3812(95)02707-L
Gedanitz, H., Davila, M. J., & Lemmon, E. W. (2015). Speed of sound measurements and a fundamental equation of state for cyclopentane. Journal of Chemical & Engineering Data, 60(5), 1331–1337. https://doi.org/10.1021/je5010164 DOI: https://doi.org/10.1021/je5010164
Grigor’ev, B., Alexandrov, I., & Gerasimov, A. (2016). Generalized equation of state for the cyclic hydrocarbons over a temperature range from the triple point to 700 K with pressures up to 100 MPa. Fluid Phase Equilibria, 418, 15–36. https://doi.org/10.1016/j.fluid.2015.07.046 DOI: https://doi.org/10.1016/j.fluid.2015.07.046
Huber, M. L., Lemmon, E. W., Bell, I. H., & McLinden, M. O. (2022). The NIST REFPROP database for highly accurate properties of industrially important fluids. Industrial & Engineering Chemistry Research, 61(42), 15449–15472. https://doi.org/10.1021/acs.iecr.2c01427 DOI: https://doi.org/10.1021/acs.iecr.2c01427
Bell, I. H. (2024). Superancillary Equations for the Multiparameter Equations of State in REFPROP 10.0. Journal of Physical and Chemical Reference Data, 53(1). https://doi.org/10.1063/5.0191228 DOI: https://doi.org/10.1063/5.0191228
Rulhoff S. (n.d.). Cyclopentane. Haltermann Carless Group GmbH. https://www.haltermann-carless.com/products/ cyclopentane
Lecompte, S., Huisseune, H., Van Den Broek, M., Vanslambrouck, B., & De Paepe, M. (2015). Review of organic Rankine cycle (ORC) architectures for waste heat recovery. Renewable and sustainable energy reviews, 47, 448–461. https://doi.org/10.1016/j.rser.2015.03.089 DOI: https://doi.org/10.1016/j.rser.2015.03.089
Ginosar, D. M., Petkovic, L. M., & Guillen, D. P. (2011). Thermal stability of cyclopentane as an organic Rankine cycle working fluid. Energy & Fuels, 25(9), 4138–4144. https://doi.org/10.1021/ef200639r DOI: https://doi.org/10.1021/ef200639r
Minor, B. H. (2006). Azeotropic compositions of cyclopentane. (Patent No. 7144523 B2). United States. Applicant: E. I. du Pont de Nemours and Company.
Kılıç, B., Arabacı, E., & Öz, A. (2024). Comparative Thermodynamic and Environmental Analysis of Vapor Compression Refrigeration System Using C-Pentane as Refrigerant. Scientific Journal of Mehmet Akif Ersoy University, 7(1), 36–42. https://doi.org/10.70030/sjmakeu.1473745 DOI: https://doi.org/10.70030/sjmakeu.1473745
International Organization for Standardization (2017). Explosive atmospheres — Part 20-1: Material characteristics for gas and vapour classification — Test methods and data (ISO/IEC 80079-20-1:2017, including Cor 1:2018). https://www.iso.org/standard/76568.html
Brown, L. J., Morgan, R. E., Haworth, G. J., & Beerwart, A. (1999). Comparative evaluations of alternative blowing agent systems in appliance foams and cabinets. Journal of cellular plastics, 35(2), 105–117. https://doi.org/10.1177/0021955X9903500202 DOI: https://doi.org/10.1177/0021955X9903500202
Bazzo, W., Cappella, A., & Talbot, S. (1996). Cyclopentane blown foam systems for domestic appliances application. Journal of cellular plastics, 32(1), 46–61. https://doi.org/10.1177/0021955X9603200104 DOI: https://doi.org/10.1177/0021955X9603200104
Hermama, C., Bensiali, B., Lahbabi, S., & El Maliki, A. (2023). Effect of the shape and the distribution of cells on the effective thermal conductivity of polyurethane foam. Polymer Engineering & Science, 63(7), 2278–2294. https://doi.org/10.1002/pen.26376 DOI: https://doi.org/10.1002/pen.26376
Schilling, S. L. (2000). Appliance rigid foams blown with cyclopentane and cyclopentane/isopentane blends. Journal of cellular plastics, 36(3), 190–206. https://doi.org/10.1177/0021955X0003600302 DOI: https://doi.org/10.1106/P8K6-YRWC-K5RA-BCWY
Santiago‐Calvo, M., Tirado‐Mediavilla, J., Ruiz‐Herrero, J. L., Villafañe, F., & Rodríguez‐Pérez, M. Á. (2019). Long‐term thermal conductivity of cyclopentane–water blown rigid polyurethane foams reinforced with different types of fillers. Polymer International, 68(10), 1826–1835. https://doi.org/10.1002/pi.5893 DOI: https://doi.org/10.1002/pi.5893
Corak, D., Barth, T., Høiland, S., Skodvin, T., Larsen, R., & Skjetne, T. (2011). Effect of subcooling and amount of hydrate former on formation of cyclopentane hydrates in brine. Desalination, 278(1–3), 268–274. https://doi.org/10.1016/j.desal.2011.05.035 DOI: https://doi.org/10.1016/j.desal.2011.05.035
Ho-Van, S., Bouillot, B., Douzet, J., Babakhani, S. M., & Herri, J. M. (2018). Implementing cyclopentane hydrates phase equilibrium data and simulations in brine solutions. Industrial & Engineering Chemistry Research, 57(43), 14774–14783. https://doi.org/10.1021/acs.iecr.8b02796 DOI: https://doi.org/10.1021/acs.iecr.8b02796
Zhang, J., Chen, S., Mao, N., & He, T. (2022). Progress and prospect of hydrate-based desalination technology. Frontiers in Energy, 1–15. https://doi.org/10.1007/s11708-021-0740-5 DOI: https://doi.org/10.1007/s11708-021-0740-5
Mottet, B. (2019). Method for crystallising clathrates hydrates, and method for purifying an aqueous liquid using the clathrates hydrates thus crystallised. (Patent No. US10501339 B2). United States. Applicant: BGH.
Ho-Van, S., Bouillot, B., Douzet, J., Babakhani, S. M., & Herri, J. M. (2019). Cyclopentane hydrates — A candidate for desalination? Journal of Environmental Chemical Engineering, 7(5), 103359. https://doi.org/10.1016/j.jece.2019.103359 DOI: https://doi.org/10.1016/j.jece.2019.103359
Connor, D. S. (1969). Process for the preparation of cyclic alkanes. (Patent No. 3450782 А). United States. Applicant: Procter and Gamble Co.
Ahluwalia, V. K., & Aggarwal, R. (2023). Alicyclic Chemistry. Springer. https://doi.org/10.1007/978-3-031-36068-8 DOI: https://doi.org/10.1007/978-3-031-36068-8
Silverman, G. S., & Rakita, P. E. (1996). Handbook of Grignard reagents. CRC Press. https://doi.org/10.1201/b16932 DOI: https://doi.org/10.1201/b16932
Thorpe, G.A.R.K. (1925). Organic syntheses. Vol. V Cyclopentanone. Journal of the Society of Chemical Industry. https://doi.org/10.15227/orgsyn.005.0037 DOI: https://doi.org/10.15227/orgsyn.005.0037
Brewster, J. H. (1954). Reductions at metal surfaces. II. A Mechanism for the Clemmensen Reduction1. Journal of the American Chemical Society, 76(24), 6364–6368. https://doi.org/10.1021/ja01653a035 DOI: https://doi.org/10.1021/ja01653a035
Wu, L., Pu, X., & Liu, Y. (2017). Solvent Screening for Cyclopentane Purification Based on COSMO. International Journal of Chemical Engineering and Applications, 8(2), 97. https://doi.org/10.18178/ijcea.2017.8.2.637 DOI: https://doi.org/10.18178/ijcea.2017.8.2.637
Ren, H., & Li, W. (2025). Study of the Salt-Adding Extractive Distillation of Cyclopentane-2, 2-dimethylbutane Using Composite Extract for the Production of High-Pure Cyclopentane. Petroleum Chemistry, 65(1), 82–92. https://doi.org/10.1134/S0965544124601947 DOI: https://doi.org/10.1134/S0965544124601947
Miki, H. (2019). Development of process for production of highly valuable chemicals derived from dicyclopentadiene for comprehensive utilization of C5 chemicals. Journal of the Japan Petroleum Institute, 62(6), 245–254. https://doi.org/10.1627/jpi.62.245 DOI: https://doi.org/10.1627/jpi.62.245
Raud, É. A., Lioznov, M. A., Yushina, E. Y., & Smidovich, E. V. (1989). Kinetics of coke deposition in pyrolysis of gasoline fractions. Chemistry and Technology of Fuels and Oils, 25(1), 19-23. https://doi.org/10.1007/BF00725202 DOI: https://doi.org/10.1007/BF00725202
Horsley, L. H. (1947). Table of azeotropes and nonazeotropes. Analytical Chemistry, 19(8), 508–600. http://doi.org/10.1021/ac60031a022 DOI: https://doi.org/10.1021/ac60008a002
Yamazaki, M. (2004). Industrialization and application development of cyclo-olefin polymer. Journal of Molecular Catalysis A: Chemical, 213(1), 81–87. https://doi.org/10.1016/j.molcata.2003.10.058 DOI: https://doi.org/10.1016/j.molcata.2003.10.058
Hsu, H. C., Wang, S. J., Ou, J. D. Y., & Wong, D. S. H. (2015). Simplification and intensification of a C5 separation process. Industrial & Engineering Chemistry Research, 54(40), 9798–9804. https://doi.org/10.1021/acs.iecr.5b01705 DOI: https://doi.org/10.1021/acs.iecr.5b01705
Junyuan Petroleum Group. (n.d.). Junyuan Petroleum Group. https://junyuanpetroleumgroup.com/
Kanne, U., Heners, J., & Krug, T. (2000). (Patent No. 6153804 A). United States. Applicant: BASF SE.
Haltermann Carless Group GmbH. (n.d.). Hydrogenation units, Speyer. https://www.haltermann-carless.com/de/hydrierung-speyer
Arutyunov, I. A., Kulik, A. V., Khakhin, L. A., & Pominova, G. S. (2018). Sposob polucheniya tsiklopentana [Method for producing cyclopentane] (Patent No. 2659227 C1). Russian Federation. Applicant: Publichnoe aktsionernoe obshchestvo "Neftyanaya kompaniya "Rosneft" (PAO "NK "Rosneft"). [in Russian].
Sharifullin, I. G., Sakhabutdinov, A. G., Amirkhanov, A. T., Misbakhov, I. R., Belanogov, I. A., Shepelin, V. A., & Gilmullin, R. R. (2017). Sposob polucheniya tsiklopentana [Method for producing cyclopentane] (Patent No. 2618233 C1). Russian Federation. Applicant: Publichnoe aktsionernoe obshchestvo “Nizhnekamskneftekhim” [in Russian].
Wan, Sh., Wang, M., Ye, G., & Chen, Y. (2000). Preparation method of cyclopentane. (Patent No. 1321625 A). China. Applicant: Sinopec Research Institute of Petroleum Processing, China Petrochemical Corp [in Chinese].
Tadao, M. & Takashi, О., (2004). Method for producing high-purity cyclopentane. (Patent No. 2004323485 A). Japan. Applicant: Idemitsu Petrochemical Co Ltd [in Japanese].
High purity production technology of Cyclopentane (Jun 23, 2022). Heze Sirloong Chemical. https://ru.sirloonggas.com/info/high-purity-production-technology-of-cyclopent-73202636.html
Ziyatdinov, A. Sh., Maltsev, L. V., Sadrieva, F. M., Boreyko, N. P., Gavrilov, G. S., Vafina, S. F., Elizarov, V. I. (2003). Sposob vydeleniya tsiklopentana [Method for isolating cyclopentane] (Patent No. 2220128 C1). Russian Federation. Applicant: Otkrytoe aktsionernoe obshchestvo “Nizhnekamskneftekhim” [in Russian].
Deng, Q., Zhang, X., Wang, L., & Zou, J. J. (2015). Catalytic isomerization and oligomerization of endo-dicyclopentadiene using alkali-treated hierarchical porous HZSM-5. Chemical Engineering Science, 135, 540–546. https://doi.org/10.1016/j.ces.2014.08.060 DOI: https://doi.org/10.1016/j.ces.2014.08.060
Herink, T., Fulín, P., Krupka, J., & Pašek, J. (2022). New Technology for Production of Dicyclopentadiene and Methyl-Dicyklopentadiene. Polymers, 14(4), 667. https://doi.org/10.3390/polym14040667 DOI: https://doi.org/10.3390/polym14040667
Kim, H. G., Lee, H. R., Lim, C. S., & Seo, B. (2019). Separation of Dicyclopentaidene in a C5 stream using a tetraethoxydimethyl disiloxane-derived silica composite membrane. Journal of Industrial and Engineering Chemistry, 79, 79–86. https://doi.org/10.1016/j.jiec.2019.05.016 DOI: https://doi.org/10.1016/j.jiec.2019.05.016
Schwerdtel, W. (1970). Process for the preparation of cyclopentene from cyclopentadiene. (Patent No. 2025411 A1). Germany. Applicant: Bayer [in German].
Schliebs, R., Brandt, H. W., Engelhard, B., Steude, H., Scherb, H., & Schnuchel, G. (1972). Process for recovering cyclopentene, isoprene and a diolefin stream from the C5-cut obtained by petroleum cracking. (Patent No. 3686349 A). Germany. Applicant: Bayer AG, Erdoelchemie GmbH.
Guo, S., Zhou, F., Fan, C., & Gu, C. (2005). Method of preparing cyclopentane by continuous hydrogenation of cyclopentadiene. (Patent No. 1911875 A). China. Applicant: China Petroleum and Chemical Corp, Sinopec Shanghai Petrochemical Co Ltd. [in Chinese].
Lattner, J., McMullen, H., Sanchez, L., Silverberg, S., & Dennis, Wu T. (1999). Process for forming cyclopentane from dicyclopentadiene. (Patent No. 5998683 A). United States. Applicant: ExxonMobil Chemical Patents Inc.
Tabler, D. (1974). Hydrogenation of cyclopentadiene. (Patent No. 3853748 A). United States. Applicant: Phillips Petroleum Co.
GUOShizhuo, X., XIARonghui, Z. (2005). Kinetics on hydrogenation of cyclopentadiene over Pd/γ-Al2O3 catalyst. Chinese Journal of Chemical Engineering, 13(5), 623. https://cjche.cip.com.cn/EN/Y2005/V13/I5/623
Wang, W. J., Qiao, M. H., Yang, J., Xie, S. H., & Deng, J. F. (1997). Selective hydrogenation of cyclopentadiene to cyclopentene over an amorphous NiB/SiO2 catalyst. Applied Catalysis A: General, 163(1–2), 101–109. https://doi.org/10.1016/S0926-860X(97)00125-7 DOI: https://doi.org/10.1016/S0926-860X(97)00125-7
Kaminsky, W. (2004). Polymerisation Catalysis. In Basic Principles in Applied Catalysis (pp. 403-440). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-662-05981-4_11 DOI: https://doi.org/10.1007/978-3-662-05981-4_11
Nishimura, S. (2001). Handbook of heterogeneous catalytic hydrogenation for organic synthesis (pp. 213–215). New York: Wiley. https://doi.org/10.1021/op0100798 DOI: https://doi.org/10.1021/op0100798
Raoult, Y., Choukroun, R., Basso-Bert, M., & Gervais, D. (1992). Hydrogenation and isomerization of olefins with diphenylphosphinomethyl hydride zirconium, [Cp2ZrH(CH2PPh2)]n, a selective homogeneous catalyst. Journal of molecular catalysis, 72(1), 47–58. https://doi.org/10.1016/0304-5102(92)80029-G DOI: https://doi.org/10.1016/0304-5102(92)80029-G
Silverberg, S. E., Sanchez, L. E., & Lattner, J. R. (2000). Use of catalytic distillation to produce cyclopentane or cyclopentene. (Patent No. 6100435 A). United States. Applicant: ExxonMobil Chemical Patents Inc.
Baiguzin, F. A., Burmistrov, D. A., Irdinkin, S. A., & Filina, M. P. (2018). Synthesis of Cyclopentane from Dicyclopentadiene under Conditions of Concurrent Downward Flow in the Catalytic Zone of a Reactive Distillation Units. Kataliz v promyshlennosti, 18(1), 6–12. https://doi.org/10.18412/1816-0387-2018-1-6-12 DOI: https://doi.org/10.18412/1816-0387-2018-1-6-12
Ai, F., Yang, L., Qiao, K., Fangm, X., Xu, T., Qi, Wenbo & et al. (2018). A kind of method that high-purity cyclopentadiene is prepared by dicyclopentadiene. (Patent No 108069813 A). China. Applicant: China Petroleum and Chemical Corp, Sinopec Fushun Research Institute of Petroleum and Petrochemicals. [in Chinese].
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Mariia P. Filina, Abdigali A. Bakibaev, Farkhad A. Baiguzin

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



