Mn(II) and Zn(II) Complexes of a Coumarin Derivative: Synthesis, Characterization and Biological Potential
DOI:
https://doi.org/10.31489/2959-0663/4-25-11Keywords:
coumarin derivatives, manganese, zinc metal complexes, antioxidant activity, antibacterial activity, molecular docking, X-ray diffraction, thermal analysis, antidiabeticAbstract
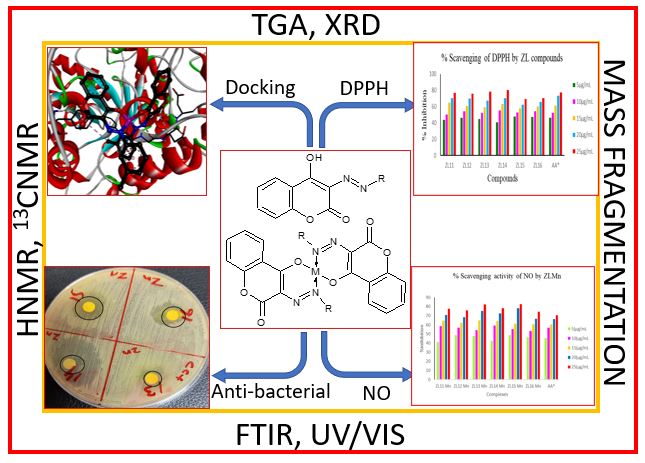
This study focused on the synthesis, characterization and biological evaluation of coumarin derivatives and their metal complexes. The synthesized compounds were characterized using spectroscopic techniques, including FTIR, 1H NMR, 13C NMR, X-ray diffraction (XRD), and thermal analysis, with mass spectrometry confirming their molecular weights. Notably, the XRD analysis revealed crystal sizes of 4.092 nm for ZL16, 4.34 nm for ZL16Mn, and 1.57 nm for ZL16Zn. FTIR analysis confirmed the presence of the –N=N– group, and comparative UV-Visible spectra validated the successful synthesis of new coumarin compounds. Antibacterial activity of synthesized compounds was evaluated against gram-negative and gram-positive bacteria using the disc diffusion method, with inhibition zones ranging from 7–31 mm, compared to the standard drug Amikacin, which had a zone of 15 mm. Antioxidant activity was assessed with IC50 values between 5.65–11.84 µg/mL for DPPH and 5.89–11.20 µg/mL for NO, compared to ascorbic acid. Molecular Docking analysis revealed strong binding interactions between the synthesized compounds and the Mannosyl-oligosaccharide glucosidase and Oligo-1,6-glucosidase enzymes, with binding energies ranging from –9.8 to –10.9 kcal/mol. These findings contribute to the field of medicinal chemistry, highlighting the potential of these compounds as therapeutic agents. Further investigations are required at molecular level to explore their full therapeutic potential.
References
Abdel-Latif, S. A., & Moustafa, H. (2018). Synthesis, spectroscopic properties, density functional theory calculations, and nonlinear optical properties of novel complexes of 5-hydroxy-4,7-dimethyl-6-(phenylazo) coumarin with Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) metal ions. Applied Organometallic Chemistry, 32(4), e4269. https://doi.org/10.1002/aoc.4269 DOI: https://doi.org/10.1002/aoc.4269
Adimule, V. M., Nandi, S. S., Kerur, S., Khadapure, S. A., & Chinnam, S. (2022). Recent advances in the one-pot synthesis of coumarin derivatives from different starting materials using nanoparticles: A review. Topics in Catalysis, 1-31. https://doi.org/10.1007/s11244-022-01554-0 DOI: https://doi.org/10.1007/s11244-022-01571-z
Anderson, N. C., Hendricks, M. P., Choi, J. J., & Owen, J. S. (2013). Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: Spectroscopic observation of facile metal-carboxylate displacement and binding. Journal of the American Chemical Society, 135(49), 18536–18548. https://doi.org/10.1021/ja4091298 DOI: https://doi.org/10.1021/ja4086758
Attaullah, H. M., Ejaz, S. A., Channar, P. A., Saeed, A., Ujan, R., Zargar, S., Channar, S. A., Sahito, R., Wani, T. A., & Abbas, Q. (2024). Exploration of newly synthesized azo-thiohydantoins as potential alkaline phosphatase inhibitors via advanced biochemical characterization and molecular modeling approaches. BMC Chemistry, 18(1), 47. https://doi.org/10.1186/s13065-024-00472-3 DOI: https://doi.org/10.1186/s13065-024-01149-8
Balewski, Ł., Szulta, S., Jalińska, A., & Kornicka, A. (2021). A mini-review: Recent advances in coumarin-metal complexes with biological properties. Frontiers in Chemistry, 9, 781779. https://doi.org/10.3389/fchem.2021.781779 DOI: https://doi.org/10.3389/fchem.2021.781779
Bazargani, M. M., & Rohloff, J. (2016). Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control, 61, 156–164. https://doi.org/10.1016/j.foodcont.2015.09.036 DOI: https://doi.org/10.1016/j.foodcont.2015.09.036
Catapano, M. C., Karlíčková, J., Tvrdý, V., Sharma, S., Prasad, A. K., Saso, L., Chhillar, A. K., Kuneš, J., Pour, M., & Parmar, V. S. (2018). Mono and dihydroxy coumarin derivatives: Copper chelation and reduction ability. Journal of Trace Elements in Medicine and Biology, 46, 88–95. https://doi.org/10.1016/j.jtemb.2017.10.003 DOI: https://doi.org/10.1016/j.jtemb.2017.11.014
Filipsky, T., Riha, M., Macakova, K., Anzenbacherová, E., Karlickova, J., & Mladenka, P. (2015). Antioxidant effects of coumarins include direct radical scavenging, metal chelation, and inhibition of ROS-producing enzymes. Current Topics in Medicinal Chemistry, 15(5), 415–431. https://doi.org/10.2174/1568026614666150407124745 DOI: https://doi.org/10.2174/1568026615666150206152233
Garg, S. S., Gupta, J., Sharma, S., & Sahu, D. (2020). An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. European Journal of Pharmaceutical Sciences, 152, 105424. https://doi.org/10.1016/j.ejps.2020.105424 DOI: https://doi.org/10.1016/j.ejps.2020.105424
Ghanghas, P., Choudhary, A., Kumar, D., & Poonia, K. (2021). Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorganic Chemistry Communications, 130, 108710. https://doi.org/10.1016/j.inoche.2021.108710 DOI: https://doi.org/10.1016/j.inoche.2021.108710
Gupta, A., Mumtaz, S., Li, C.-H., Hussain, I., & Rotello, V. M. (2019). Combatting antibiotic-resistant bacteria using nanomaterials. Chemical Society Reviews, 48(2), 415–427. https://doi.org/10.1039/C7CS00857J DOI: https://doi.org/10.1039/C7CS00748E
Houas, N., Chafaa, S., Chafai, N., Ghedjati, S., Djenane, M., & Kitouni, S. (2022). Synthesis, characterization, DFT study, and antioxidant activity of (2-hydroxynaphthalen-1-yl)methyl-2-hydroxyphenylamino phosphonic acid. Journal of Molecular Structure, 1247, 131322. https://doi.org/10.1016/j.molstruc.2021.131322 DOI: https://doi.org/10.1016/j.molstruc.2021.131322
Kadhum, A. A. H., Al-Amiery, A. A., Musa, A. Y., & Mohamad, A. B. (2011). The antioxidant activity of new coumarin derivatives. International Journal of Molecular Sciences, 12(9), 5747–5761. https://doi.org/10.3390/ijms12095747 DOI: https://doi.org/10.3390/ijms12095747
Kapur, A., Hasković, A., Čopra-Janićijević, A., Klepo, L., Topčagić, A., Tahirović, I., & Sofić, E. (2012). Spectrophotometric analysis of total ascorbic acid content in various fruits and vegetables. Bulletin of the Chemists and Technologists of Bosnia and Herzegovina, 38(4), 39–42. https://doi.org/10.35773/bctbh.2012.38.4.39
Kaur, H., Lim, S. M., Ramasamy, K., Vasudevan, M., Shah, S. A. A., & Narasimhan, B. (2020). Diazenyl Schiff bases: Synthesis, spectral analysis, antimicrobial studies, and cytotoxic activity on human colorectal carcinoma cell line (HCT-116). Arabian Journal of Chemistry, 13(1), 377–392. https://doi.org/10.1016/j.arabjc.2016.08.004 DOI: https://doi.org/10.1016/j.arabjc.2017.05.004
Keri, R. S., Sasidhar, B., Nagaraja, B. M., & Santos, M. A. (2015). Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. European Journal of Medicinal Chemistry, 100, 257–269. https://doi.org/10.1016/j.ejmech.2015.06.037 DOI: https://doi.org/10.1016/j.ejmech.2015.06.017
Kharadi, G. (2012). Thermal decomposition and mass spectra of mixed ligand copper (II) complexes of 1,10-phenanthroline and coumarin derivatives. Journal of Thermal Analysis and Calorimetry, 107(2), 651–659. https://doi.org/10.1007/s10973-011-1760-2 DOI: https://doi.org/10.1007/s10973-011-1671-x
Lončarić, M., Gašo-Sokač, D., Jokić, S., & Molnar, M. (2020). Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules, 10(1), 151. https://doi.org/10.3390/biom10010151 DOI: https://doi.org/10.3390/biom10010151
Maobe, M. A., & Nyarango, R. M. (2013). Fourier transformer infra-red spectrophotometer analysis of Urtica dioica medicinal herb used for the treatment of diabetes, malaria, and pneumonia in Kisii region, Southwest Kenya. World Applied Sciences Journal, 21(8), 1128–1135. https://doi.org/10.5829/idosi.wasj.2013.21.8.1898
Marchi, R. C., Campos, I. A., Santana, V. T., & Carlos, R. M. (2022). Chemical implications and considerations on techniques used to assess the in vitro antioxidant activity of coordination compounds. Coordination Chemistry Reviews, 451, 214275. https://doi.org/10.1016/j.ccr.2022.214275 DOI: https://doi.org/10.1016/j.ccr.2021.214275
Ndagi, U., Mhlongo, N., & Soliman, M. E. (2017). Metal complexes in cancer therapy–an update from a drug design perspective. Drug Design, Development and Therapy, 599–616. https://doi.org/10.2147/DDDT.S134156 DOI: https://doi.org/10.2147/DDDT.S119488
Olson, E. J., & Bülmann, P. (2011). Getting more out of a Job plot: Determination of reactant to product stoichiometry in cases of displacement reactions and n:n complex formation. The Journal of Organic Chemistry, 76(20), 8406–8412. https://doi.org/10.1021/jo2015719 DOI: https://doi.org/10.1021/jo201624p
Patil, S. A., Unki, S. N., & Badami, P. S. (2013). Synthesis, characterization, biological and thermal behavior of Co(II), Ni(II), and Cu(II) complexes with Schiff bases having coumarin moieties. Journal of Thermal Analysis and Calorimetry, 111, 1281–1289. https://doi.org/10.1007/s10973-012-2809-2 DOI: https://doi.org/10.1007/s10973-012-2293-7
Peng, X.-M., LV Damu, G., & Zhou, H. (2013). Current developments of coumarin compounds in medicinal chemistry. Current Pharmaceutical Design, 19(21), 3884–3930. https://doi.org/10.2174/1381612811319210010 DOI: https://doi.org/10.2174/1381612811319210013
Retnam, C. G., Rose, S. V., & Kumari, B. S. (2023). Synthesis, characterization, biological activity, and molecular docking study of transition metal complexes from heterocyclic ligand system. Journal of Molecular Structure, 1282, 135162. https://doi.org/10.1016/j.molstruc.2023.135162 DOI: https://doi.org/10.1016/j.molstruc.2023.135162
Sharma, T., Singh, D., Mahapatra, A., Mohapatra, P., Sahoo, S., & Sahoo, S. K. (2022). Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. OpenNano, 100074. https://doi.org/10.1016/j.opnano.2022.100074 DOI: https://doi.org/10.1016/j.onano.2022.100074
Sohrabi, M., Binaeizadeh, M. R., Iraji, A., Larijani, B., Saeedi, M., & Mahdavi, M. (2022). A review on α-glucosidase inhibitory activity of first-row transition metal complexes: A futuristic strategy for the treatment of type 2 diabetes. RSC Advances, 12(19), 12011–12052. https://doi.org/10.1039/D2RA02383A DOI: https://doi.org/10.1039/D2RA00067A
Souhangir, M., Bidoki, S. M., & Gharanjig, K. (2022). Synthesis of a novel fluorescent reactive dye based on coumarin-benzimidazole for high visibility dyeing of cotton. Progress in Color, Colorants and Coatings, 15(4), 327–340. https://doi.org/10.1515/pccc-2022-0024
Sunitha, N., Raj, C. I. S., & Kumari, B. S. (2023). Synthesis, spectral studies, biological evaluation, and molecular docking studies of metal complexes from coumarin derivative. Journal of Molecular Structure, 1285, 135443. https://doi.org/10.1016/j.molstruc.2023.135443 DOI: https://doi.org/10.1016/j.molstruc.2023.135443
Wiester, M. J., Ulmann, P. A., & Mirkin, C. A. (2011). Enzyme mimics based upon supramolecular coordination chemistry. Angewandte Chemie International Edition, 50(1), 114–137. https://doi.org/10.1002/anie.201004563 DOI: https://doi.org/10.1002/anie.201000380
Zaidan, M., Noor Rain, A., Badrul, A., Adlin, A., Norazah, A., & Zakiah, I. (2005). In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed, 22(2), 165–170.
Zhang, G., Zheng, H., Guo, M., Du, L., Liu, G., & Wang, P. (2016). Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener, and surface sizing agent. Applied Surface Science, 367, 167–173. https://doi.org/10.1016/j.apsusc.2016.01.031 DOI: https://doi.org/10.1016/j.apsusc.2016.01.110
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Zulfiqar Ali Shahid, Rukhsana Tabassum

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



