Bioactive Chitosan/β-Tricalcium Phosphate Coatings on Titanium: Experimental Optimization and DFT Insight
DOI:
https://doi.org/10.31489/2959-0663/4-25-12Keywords:
chitosan, Bombyx mori, DFT modeling, chitosan coating, β-tricalcium phosphate, TiO2(110), electrochemical deposition, osteogenesis, implantAbstract
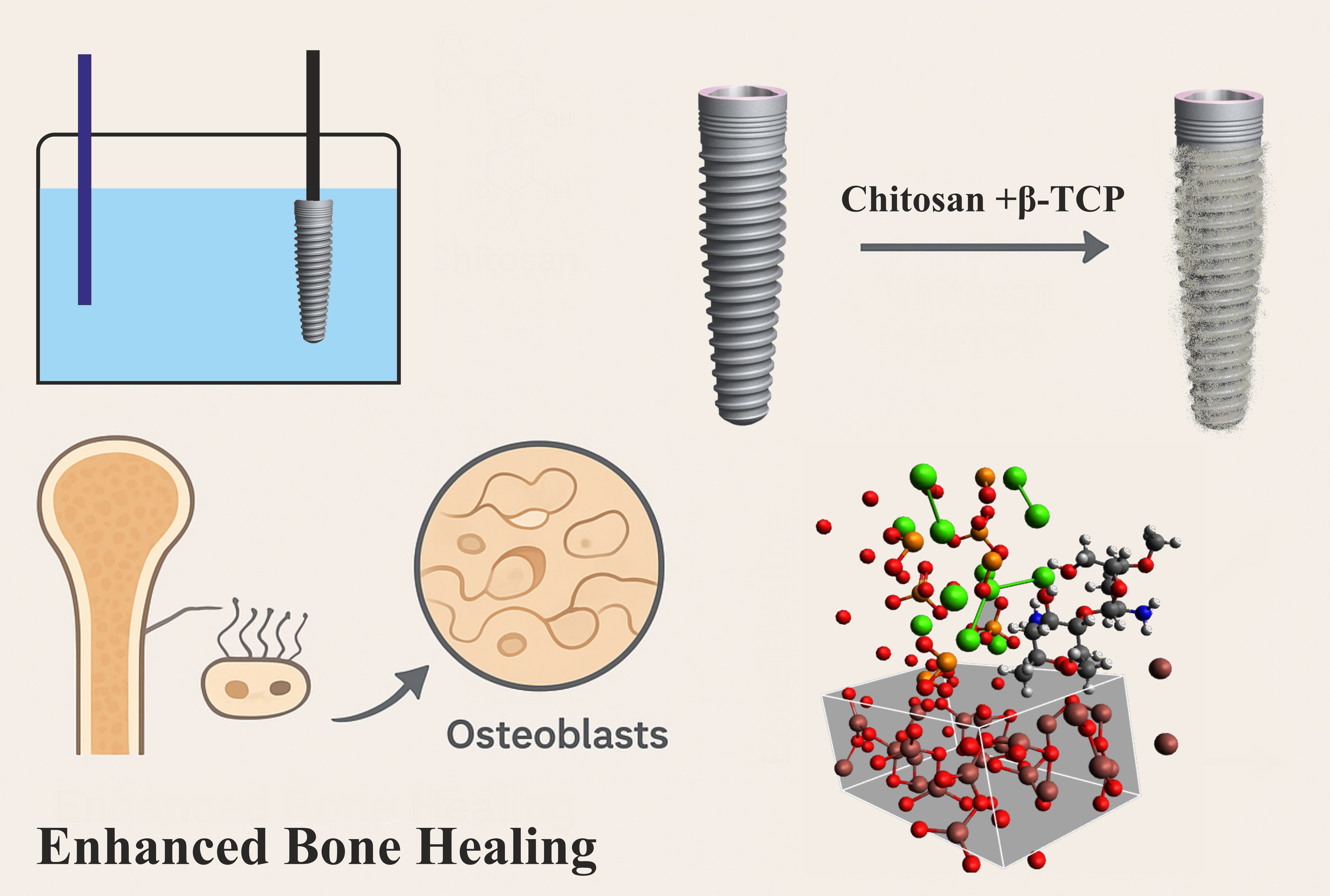
The development of bioactive coatings combining biopolymers and calcium phosphate ceramics offers an effective strategy to improve osseointegration and functional performance of titanium-based implants. In this study, highly purified Bombyx mori chitosan (CS-BM) and β-tricalcium phosphate (β-TCP) were used to fabricate hybrid coatings on titanium substrates via electrochemical deposition under optimized conditions (52 °C, pH 6.6–7.0, 2.0–4.0 mA·cm–2). SEM and AFM analyses revealed uniform, strongly adherent two-layer structures with microtopography (1–10 μm roughness) favorable for bone ingrowth, while elemental analysis confirmed complete Ca- and P-rich coverage. Density functional theory (DFT) modeling confirmed the cooperative role of Ca2+ and PO43– in bridging CS-BM to TiO2, contributing to coating stability. In vivo studies demonstrated that CS-BM/TCP-coated implants accelerated early contact osteogenesis, dense trabecular bone formation, and stable bone–implant integration compared to uncoated controls. In an experimental osteoporosis model, intramuscular administration of CS-BM with active calcium or calcium–vitamin D3 significantly enhanced fibroblast-to-osteoblast differentiation, stimulated osteoid synthesis, and led to complete restoration of bone microarchitecture within 21 days. These findings highlight CS-BM/TCP coatings as a sustainable, locally sourced, and highly effective platform for orthopedic and dental implant applications, with additional therapeutic potential in osteoporosis management.
References
Raj, H.K.G., Sadasivam, G., & Dommeti, V.K. (2025). Biocomposites-coated biodegradable materials with optimized properties for orthopedic implant biodegradability and performance: A comparative study. ACS Applied Bio Materials, 8(6), 5276–5290. https://doi.org/10.1021/acsabm.5c00603 DOI: https://doi.org/10.1021/acsabm.5c00603
Ma, R., Wu, Z., Guo, X., Wu, Z., Zhu, Z., Qu, Y., Wang, K., Li, C., Ma, K., & Yang, P. (2025). A dual-functional biodegradable composite coating fabricated on sulfonated PEEK via vacuum cold spraying: Immunomodulation-driven osteointegration. Journal of Materials Chemistry B, 13, 7155–7171. https://doi.org/10.1039/D5TB00628G DOI: https://doi.org/10.1039/D5TB00628G
Du, W., Guo, X., Zheng, Q., Zhang, Y., Li, H., & Wang, S. (2025). Development of a biodegradable α-TCP/PLA/nMgO composite for enhanced guided bone regeneration. Scientific Reports, 15, 19675. https://doi.org/10.1038/s41598-025-03426-5 DOI: https://doi.org/10.1038/s41598-025-03426-5
Xie, Y., Tan, J., Fang, S., Li, T., Chen, Y., Li, L., & Chen, N. (2024). A biodegradable, osteo-regenerative and biomechanically robust polylactide bone screw for clinical orthopedic surgery. International Journal of Biological Macromolecules, 283, 137477. https://doi.org/10.1016/j.ijbiomac.2024.137477 DOI: https://doi.org/10.1016/j.ijbiomac.2024.137477
Jiménez-Morales, A., Solís-Garrido, Á., Toirac, B., Martínez, P., Alonso, M., & Rodríguez, J. (2025). Bilayer sol-gel system for local prevention in prosthetic joint infections and osteointegration improvement. Communications Materials, 6, 67. https://doi.org/10.1038/s43246-025-00790-7 DOI: https://doi.org/10.1038/s43246-025-00790-7
Liu, W.C., Chang, H.W., Huang, S.I., Lin, C.H., & Wu, Y.C. (2025). Biodegradable porous iron versus titanium interference screws in porcine ACL reconstruction model: A one-year observational study. Materials Degradation, 9, 77. https://doi.org/10.1038/s41529-025-00602-w DOI: https://doi.org/10.1038/s41529-025-00602-w
Zhang, X., Jiang, W., Wu, X., Zhao, Y., Qiu, Y., & Wang, L. (2025). Divide-and-conquer strategy with engineered ossification center organoids for rapid bone healing through developmental cell recruitment. Nature Communications, 16, 6200. https://doi.org/10.1038/s41467-025-61619-y DOI: https://doi.org/10.1038/s41467-025-61619-y
Kamal, A.F., Dionysios, E., Supriadi, S., Rahmat, A., Widodo, A., & Arifin, M. (2025). Biodegradability and biocompatibility test of magnesium carbonate apatite composite implants fabricated by extrusion technique on Sprague Dawley rats. Scientific Reports, 15, 9976. https://doi.org/10.1038/s41598-025-88983-5 DOI: https://doi.org/10.1038/s41598-025-88983-5
Zhu, S., Sun, H., Mu, T., & Richel, A. (2025). Research progress in 3D printed biobased and biodegradable polyester/ceramic composite materials: Applications and challenges in bone tissue engineering. ACS Applied Materials & Interfaces, 17(2), 2791–2813. https://doi.org/10.1021/acsami.4c15719 DOI: https://doi.org/10.1021/acsami.4c15719
Xia, J., Yu, J., Shi, W., Zhou, Y., Zhang, B., & Wang, Y. (2025). Molybdenum facilitates PDLSC-based bone regeneration through the JAK/STAT3 signaling pathway. Scientific Reports, 15, 22204. https://doi.org/10.1038/s41598-025-07298-7 DOI: https://doi.org/10.1038/s41598-025-07298-7
El-Saadony, M.T., Saad, A.M., Alkafaas, S.S., Dladla, M., Ghosh, S., Elkafas, S.S., Hafez, W., Ezzat, S.M., Khedr, S.A., Hussien, A.M., Fahmy, M.A., Elesawi, I.E., Salem, H.M., Mohammed, D.M., Abd El-Mageed, T.A., Ahmed, A.E., Mosa, W.F.A., El-Tarabily, M.K., AbuQamar, S.F., & El-Tarabily, K.A. (2025). Chitosan, derivatives, and its nanoparticles: Preparation, physicochemical properties, biological activities, and biomedical applications — A comprehensive review. International Journal of Biological Macromolecules, 313, 142832. https://doi.org/10.1016/j.ijbiomac.2025.142832 DOI: https://doi.org/10.1016/j.ijbiomac.2025.142832
Soontorntepwarakul, N., Boonyarattanakalin, K., Fukasem, P., Somkhuan, S., Srirussamee, K., Choowongkomon, K., & Gleeson, M.P. (2025). Assessment of the utility of chitosan nanoparticles and microfibers in drug delivery applications of sulfamethoxazole and ciprofloxacin. New Journal of Chemistry, 49, 10047–10055. https://doi.org/10.1039/D4NJ05421K DOI: https://doi.org/10.1039/D4NJ05421K
Koirala, P., Bhattarai, P., Sriprablom, J., Zhang, R., Nirmal, S., & Nirmal, N. (2025). Recent progress of functional nano-chitosan in pharmaceutical and biomedical applications: An updated review. International Journal of Biological Macromolecules, 285, 138324. https://doi.org/10.1016/j.ijbiomac.2024.138324 DOI: https://doi.org/10.1016/j.ijbiomac.2024.138324
Pérez-Pacheco, Y., Tylkowski, B., & García-Valls, R. (2025). Chitosan micro/nanocapsules in action: Linking design, production, and therapeutic application. Molecules, 30, 252. https://doi.org/10.3390/molecules30020252 DOI: https://doi.org/10.3390/molecules30020252
Milusheva, R., & Rashidova, S. (2019). Bombyx mori chitosan nanoparticles: Synthesis and properties. Open Journal of Organic Polymer Materials, 9, 63–73. https://doi.org/10.4236/ojopm.2019.94004 DOI: https://doi.org/10.4236/ojopm.2019.94004
Milusheva, R.Yu., & Rashidova, S.Sh. (2022). Obtaining chitosan nanoparticles from Bombyx mori. Russian Chemical Bulletin, 71, 232–239. https://doi.org/10.1007/s11172-022-3402-9 DOI: https://doi.org/10.1007/s11172-022-3402-9
Pirniyazov, K., Nurgaliev, I., & Rashidova, S. (2023). Reaction of the formation of chitosan nanoascorbate Bombyx mori and computer simulation of its structure. AIP Conference Proceedings, 2931, 060002. https://doi.org/10.1063/5.0182628 DOI: https://doi.org/10.1063/5.0182628
Sagala, Y.G., Andadari, L., Handayani, T.H., Sholikin, M.M., Fitri, A., Fidriyanto, R., Rohmatussolihat, R., Ridwan, R., Astuti, W.D., Widyastuti, Y., Fassah, D.M., Wijayanti, I., & Sarwono, K.A. (2024). The effect of silkworms (Bombyx mori) chitosan on rumen fermentation, methanogenesis, and microbial population in vitro. Veterinary World, 17(6), 1216–1226. https://doi.org/10.14202/vetworld.2024.1216-1226 DOI: https://doi.org/10.14202/vetworld.2024.1216-1226
Avazova, O.B., Milusheva, R.Y., Nurgaliev, I.N., & Rashidova, S.Sh. (2022). Polymolecular complexes of chitosan with the Bombyx mori protein. Bulletin of the University of Karaganda — Chemistry, 107(3), 87–101. https://doi.org/10.31489/2022Ch3/3-22-22 DOI: https://doi.org/10.31489/2022Ch3/3-22-22
Vokhidova, N.R., Ergashev, K.H., & Rashidova, S.S. (2020). Hydroxyapatite–chitosan Bombyx mori: Synthesis and physicochemical properties. Journal of Inorganic and Organometallic Polymers and Materials, 30, 3357–3368. https://doi.org/10.1007/s10904-020-01649-9 DOI: https://doi.org/10.1007/s10904-020-01649-9
Vokhidova, N.R., Khudoyberdiyev, Sh.Sh., Nurgaliev, I.N., & Rashidova, S.Sh. (2023). On obtaining binary polyelectrolyte complexes of chitosan Bombyx mori with collagen. Progress on Chemistry and Application of Chitin and its Derivatives, 28, 188–203. https://doi.org/10.15259/PCACD.28.017 DOI: https://doi.org/10.15259/PCACD.28.017
Wang, J., van Apeldoorn, A., & de Groot, K. (2006). Electrolytic deposition of calcium phosphate/chitosan coating on titanium alloy: Growth kinetics and influence of current density, acetic acid, and chitosan. Journal of Biomedical Materials Research Part A, 76(3), 503–511. https://doi.org/10.1002/jbm.a.30542 DOI: https://doi.org/10.1002/jbm.a.30542
Zhu, J., Chen, X., Wang, J., Liu, D., Li, W., & Tian, Y. (2016). Hydroxyapatite/β-tricalcium phosphate composite for guiding bone tissue growth into a titanium tube in 8 mm dog tibia cavity defects. Journal of Wuhan University of Technology, Materials Science Edition, 31, 468–473. https://doi.org/10.1007/s11595-016-1393-9 DOI: https://doi.org/10.1007/s11595-016-1393-9
Zhang, T., Zhang, X., Mao, M., Li, J., Wei, T., & Sun, H. (2020). Chitosan/hydroxyapatite composite coatings on porous Ti6Al4V titanium implants: In vitro and in vivo studies. Journal of Periodontal & Implant Science, 50(6), 392–405. https://doi.org/10.5051/jpis.1905680284 DOI: https://doi.org/10.5051/jpis.1905680284
Mishchenko, O., Yanovska, A., Kosinov, O., Maksymov, D., Moskalenko, R., Ramanavicius, A., & Pogorielov, M. (2023). Synthetic calcium–phosphate materials for bone grafting. Polymers, 15, 3822. https://doi.org/10.3390/polym15183822 DOI: https://doi.org/10.3390/polym15183822
Mondal, S., Park, S., Choi, J., Vu, T.T.H., Doan, V.H.M., Vo, T.T., Lee, B., & Oh, J. (2023). Hydroxyapatite: A journey from biomaterials to advanced functional materials. Advances in Colloid and Interface Science, 321, 103013. https://doi.org/10.1016/j.cis.2023.103013 DOI: https://doi.org/10.1016/j.cis.2023.103013
Hou, X., Zhang, L., Zhou, Z., Luo, X., Wang, T., Zhao, X., Lu, B., Chen, F., & Zheng, L. (2022). Calcium phosphate-based biomaterials for bone repair. Journal of Functional Biomaterials, 13, 187. https://doi.org/10.3390/jfb13040187 DOI: https://doi.org/10.3390/jfb13040187
Haque, T. (2025). Assessment of bone regeneration around implants using different bone substitute materials. Journal of Pharmacy and Bioallied Sciences, 17(Suppl 2), S1258–S1260. https://doi.org/10.4103/jpbs.jpbs_79_25 DOI: https://doi.org/10.4103/jpbs.jpbs_79_25
Yun, J.I., Yun, S.I., Kim, J.H., Kim, D.G., & Lee, D.-W. (2025). Mediation of osseointegration, osteoimmunology, and osteoimmunologic integration by Tregs and macrophages: A narrative review. International Journal of Molecular Sciences, 26, 5421. https://doi.org/10.3390/ijms26115421 DOI: https://doi.org/10.3390/ijms26115421
Sarvaiya, B.B., Kumar, S., Pathan, M.H., Patel, P., Shah, H., & Mehta, R. (2025). The impact of implant surface modifications on the osseointegration process: An overview. Cureus, 17, e81576. https://doi.org/10.7759/cureus.81576 DOI: https://doi.org/10.7759/cureus.81576
Xu, F., Zhao, G., Gong, Y., Liang, X., Yu, M., Cui, H., Xie, L., Zhu, N., Zhu, X., Shao, X., Qi, K., Lu, B., Tu, J., & Na, S. (2025). Enhancement of osseointegration via endogenous electric field by regulating the charge microenvironments around implants. Advanced Healthcare Materials, 14, e2403388. https://doi.org/10.1002/adhm.202403388 DOI: https://doi.org/10.1002/adhm.202403388
Wang, Q., Chen, Y., Ding, H., Li, X., Zhang, S., Zhao, Y., & Guo, J. (2025). Optogenetic activation of mechanical nociceptions to enhance implant osseointegration. Nature Communications, 16, 3093. https://doi.org/10.1038/s41467-025-58336-x DOI: https://doi.org/10.1038/s41467-025-58336-x
Tao, H., Chen, K., Wang, Q., Yang, J., Liu, L., Zhang, C., & Zhang, J. (2025). Targeting lipid raft-related stomatin to ameliorate osteoporosis in preclinical models. Nature Communications, 16, 5495. https://doi.org/10.1038/s41467-025-60032-9 DOI: https://doi.org/10.1038/s41467-025-60032-9
Wang, B., Liu, Y., Wang, Z., Zhang, Y., Gao, J., Li, F., & Li, X. (2025). Osteoporosis in adjacent cervical segments exacerbates disc herniation. Scientific Reports, 15, 22901. https://doi.org/10.1038/s41598-025-06554-0 DOI: https://doi.org/10.1038/s41598-025-06554-0
Jiang, S., Xu, K., & Chen, X. (2025). Identifying modifiable factors and their causal effects on osteoporosis risk. Scientific Reports, 15, 19472. https://doi.org/10.1038/s41598-025-04455-w DOI: https://doi.org/10.1038/s41598-025-04455-w
Morin, S.N., Leslie, W.D., & Schousboe, J.T. (2025). Osteoporosis: A review. JAMA, June 30, ahead of print. https://doi.org/10.1001/jama.2025.6003 DOI: https://doi.org/10.1001/jama.2025.6003
Park, K.H., Kim, S.J., Jeong, Y.H., Moon, H.J., Song, H.J., & Park, Y.J. (2018). Fabrication and biological properties of calcium phosphate/chitosan composite coating on titanium in modified SBF. Materials Science and Engineering: C, 90, 113–118. https://doi.org/10.1016/j.msec.2018.04.060 DOI: https://doi.org/10.1016/j.msec.2018.04.060
Thi Ngo, A., Do Chi, L., Pham, H.H., Pham, S., & Duong, L. (2024). Improvement of corrosion resistance and adhesion of hydroxyapatite coating on AZ31 alloy by an anodizing intermediate layer. Journal of Applied Biomaterials & Functional Materials, 22, 22808000241271693. https://doi.org/10.1177/22808000241271693 DOI: https://doi.org/10.1177/22808000241271693
Kresse, G., & Hafner, J. (1993). Ab Initio Molecular Dynamics for Liquid Metals. Physical Review B, 47, 558–561. https://doi.org/10.1103/PhysRevB.47.558 DOI: https://doi.org/10.1103/PhysRevB.47.558
Kresse, G., & Hafner, J. (1994). Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal–amorphous-Semiconductor Transition in Germanium. Physical Review B, 49, 14251–14269. https://doi.org/10.1103/PhysRevB.49.14251 DOI: https://doi.org/10.1103/PhysRevB.49.14251
Kresse, G., & Furthmüller, J. (1996). Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Physical Review B, 54, 11169–11186. https://doi.org/10.1103/PhysRevB.54.11169 DOI: https://doi.org/10.1103/PhysRevB.54.11169
Kresse, G., & Furthmüller, J. (1996). Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Computational Materials Science, 6, 15–50. https://doi.org/10.1016/0927-0256(96)00008-0 DOI: https://doi.org/10.1016/0927-0256(96)00008-0
Perdew, J.P., Burke, K., & Ernzerhof, M. (1996). Generalized Gradient Approximation Made Simple. Physical Review Letters, 77, 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865 DOI: https://doi.org/10.1103/PhysRevLett.77.3865
Blöchl P.E. (1994). Projector Augmented-Wave Method. Physical Review B, 50 (24), 17953–17979. https://doi.org/10.1103/PhysRevB.50.17953 DOI: https://doi.org/10.1103/PhysRevB.50.17953
Monkhorst, H.J., & Pack, J.D. (1976). Special Points for Brillouin-Zone Integrations. Physical Review B, 13, 5188–5192. https://doi.org/10.1103/PhysRevB.13.5188 DOI: https://doi.org/10.1103/PhysRevB.13.5188
Leslie, M., & Gillan, N. J. (1985). The energy and elastic dipole tensor of defects in ionic crystals calculated by the supercell method. Journal of Physics C: Solid State Physics, 18, 973. https://doi.org/10.1088/0022-3719/18/5/005 DOI: https://doi.org/10.1088/0022-3719/18/5/005
Tang, W., Sanville, E., & Henkelman, G. (2009). A grid-based Bader analysis algorithm without lattice bias. Journal of Physics: Condensed Matter, 21, 084204. https://doi.org/10.1088/0953-8984/21/8/084204 DOI: https://doi.org/10.1088/0953-8984/21/8/084204
Momma, K., & Izumi, F. (2011). VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. Journal of Applied Crystallography, 44, 1272–1276. https://doi.org/10.1107/S0021889811038970 DOI: https://doi.org/10.1107/S0021889811038970
Srivastava, A.P., & Pandey, B.K. (2025). Analysis of the structural and electronic properties of TiO2 under pressure using density functional theory and equation of state. Computational Condensed Matter, 44, e01076. https://doi.org/10.1016/j.cocom.2025.e01076 DOI: https://doi.org/10.1016/j.cocom.2025.e01076
Bastami, G. F., Paknejad, Z., Jafari, M., Salehi, M., Rezai Rad, M., & Khojasteh, A. (2017). Fabrication of a three-dimensional β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles for sustained release of bone morphogenetic protein-2: Implication for bone tissue engineering. Materials Science and Engineering: C, 72, 481–491. https://doi.org/10.1016/j.msec.2016.10.084 DOI: https://doi.org/10.1016/j.msec.2016.10.084
Elimelech, R., Khoury, N., Tamari, T., Blumenfeld, I., Gutmacher, Z., & Zigdon-Giladi, H. (2019). Use of transforming growth factor-β loaded onto β-tricalcium phosphate scaffold in a bone regeneration rat calvaria model. Clinical Implant Dentistry and Related Research, 21, 593–601. https://doi.org/10.1111/cid.12775 DOI: https://doi.org/10.1111/cid.12775
Cao, Q., He, Z., Sun, W.Q., Fan, G., Zhao, J., Bao, N., & Ye, T. (2019). Improvement of calcium phosphate scaffold osteogenesis in vitro via combination of glutamate-modified BMP-2 peptides. Materials Science and Engineering: C, 96, 412–418. https://doi.org/10.1016/j.msec.2018.11.048 DOI: https://doi.org/10.1016/j.msec.2018.11.048
Garcia, D.C., Mingrone, L.E., Cavalcanti de Sá, M.J. (2022). Evaluation of osseointegration and bone healing using pure-phase β-tricalcium phosphate ceramic implant in bone critical defects: A systematic review. Frontiers in Veterinary Science, 9, 859920. https://doi.org/10.3389/fvets.2022.859920 DOI: https://doi.org/10.3389/fvets.2022.859920
Pihlman, H., Keränen, P., Paakinaho, K., Haimi, S., Törmälä, P., & Waris, T. (2018). Novel osteoconductive β-tricalcium phosphate/poly(L-lactide-co-ε-caprolactone) scaffold for bone regeneration: A study in a rabbit calvarial defect. Journal of Materials Science: Materials in Medicine, 29, 156. https://doi.org/10.1007/s10856-018-6159-9 DOI: https://doi.org/10.1007/s10856-018-6159-9
Xie, L., Yang, Y., Fu, Z., Li, Y., Shi, J., Ma, D., Liu, S., & Luo, D. (2019). Fe/Zn-modified tricalcium phosphate (TCP) biomaterials: Preparation and biological properties. RSC Advances, 9, 781–789. https://doi.org/10.1039/C8RA08453J DOI: https://doi.org/10.1039/C8RA08453J
Kim, S.-M., Yoo, K.-H., Kim, H., Kim, Y.-I., & Yoon, S.-Y. (2022). Simultaneous substitution of Fe and Sr in beta-tricalcium phosphate: Synthesis, structural, magnetic, degradation, and cell adhesion properties. Materials, 15, 4702. https://doi.org/10.3390/ma15134702 DOI: https://doi.org/10.3390/ma15134702
Kumar, P.S., KS, S.K., Grandhi, V.V., & Gupta, V. (2019). The effects of titanium implant surface topography on osseointegration: Literature review. JMIR Biomedical Engineering, 4(1), e13237. https://doi.org/10.2196/13237 DOI: https://doi.org/10.2196/13237
Jahani, B., & Wang, X. (2021). The effects of surface roughness on the functionality of Ti13Nb13Zr orthopedic implants. Biomedical Journal of Scientific & Technical Research, 38(1), 30058–30067. https://doi.org/10.26717/BJSTR.2021.38.006104 DOI: https://doi.org/10.26717/BJSTR.2021.38.006104
Romero-Serrano, M., Romero-Ruiz, M.-M., Herrero-Climent, M., Rios-Carrasco, B., & Gil-Mur, J. (2024). Correlation between implant surface roughness and implant stability: A systematic review. Dentistry Journal, 12, 276. https://doi.org/10.3390/dj12090276 DOI: https://doi.org/10.3390/dj12090276
Xing, Y., Xu, Q., Yang, S.X., Chen, C., Tang, Y., Sun, S., Zhang, L., Che, Z., & Li, X. (2016). Preservation mechanism of chitosan-based coating with cinnamon oil for fruits storage based on sensor data. Sensors, 16(7), 1111. https://doi.org/10.3390/s16071111 DOI: https://doi.org/10.3390/s16071111
Singh, T.P., Chatli, M.K., & Sahoo, J. (2015). Development of chitosan-based edible films: Process optimization using response surface methodology. Journal of Food Science and Technology, 52(5), 2530–2543. https://doi.org/10.1007/s13197-014-1318-6 DOI: https://doi.org/10.1007/s13197-014-1318-6
Jinno, T., Davy, D.T., & Goldberg, V.M. (2002). Comparison of hydroxyapatite and hydroxyapatite tricalcium-phosphate coatings. Journal of Arthroplasty, 17(7), 902–909. https://doi.org/10.1054/arth.2002.34821 DOI: https://doi.org/10.1054/arth.2002.34821
Gutiérrez-Sánchez, M., Flores-Rocha, S., Pozos-Guillén, A., Flores, H., Escobar-Barrios, V., Palestino-Escobedo, A.G., & Escobar-García, D.M. (2025). Design, characterization, and biocompatibility of chitosan–nano-hydroxyapatite/tricalcium phosphate sponges. Tissue and Cell, 94, 102804. https://doi.org/10.1016/j.tice.2025.102804 DOI: https://doi.org/10.1016/j.tice.2025.102804
Zhou, J., Ma, R., Shi, W., Lei, S., Zhang, X., Jiang, N., Lin, Y., Li, Z., & Nie, M. (2024). Zinc and chitosan-enhanced β tricalcium phosphate from calcined fetal bovine bone for mandible reconstruction. Frontiers in Bioengineering and Biotechnology, 12, 1355493. https://doi.org/10.3389/fbioe.2024.1355493 DOI: https://doi.org/10.3389/fbioe.2024.1355493
Karimipour, A., Shahgholi, M., Attaeyan, A., Viet, P.H.H., Asiri, S.A., Alfawaz, K.M., & Alogla, A.F. (2024). The effect of initial temperature on the mechanical strength of tricalcium phosphate/chitosan/silica aerogels nanocomposites using molecular dynamics simulation. Journal of the Taiwan Institute of Chemical Engineers, 164, 105682. https://doi.org/10.1016/j.jtice.2024.105682 DOI: https://doi.org/10.1016/j.jtice.2024.105682
Senthil, R. (2024). Preparation of bioscaffold supported by chitosan and nanocurcumin to promote tissue engineering. Regenerative Engineering and Translational Medicine, 10, 553–563. https://doi.org/10.1007/s40883-024-00344-2 DOI: https://doi.org/10.1007/s40883-024-00344-2
Fischer, V., Haffner-Luntzer, M., Prystaz, K., Vom Scheidt, A., Busse, B., Schinke, T., Amling, M., & Ignatius, A. (2017). Calcium and vitamin D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Scientific Reports, 7, 7223. https://doi.org/10.1038/s41598-017-07511-2 DOI: https://doi.org/10.1038/s41598-017-07511-2
Saul, D., & Khosla, S. (2022). Fracture healing in the setting of endocrine diseases, aging, and cellular senescence. Endocrine Reviews, 43, 984–1002. https://doi.org/10.1210/endrev/bnac008 DOI: https://doi.org/10.1210/endrev/bnac008
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Rakiya Yu. Milusheva, Ilnar N. Nurgaliev, Akmal B. Abilkasimov , Sayora Sh. Rashidova

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



