Features on the Way to the Synthesis of 1-Benzoyl-2-Phenyl-3a, 6a-Diazapentalene and 1-Pivaloyl-2-Tert-Butyl-3a,6a-Diazapentalene
DOI:
https://doi.org/10.31489/2959-0663/4-25-17Keywords:
3a,6a-diazapentalenes, NMR, Aldol, cyclocondensation, quaternization, pyrazole, N-acylalkylation, α-bromoketonesAbstract
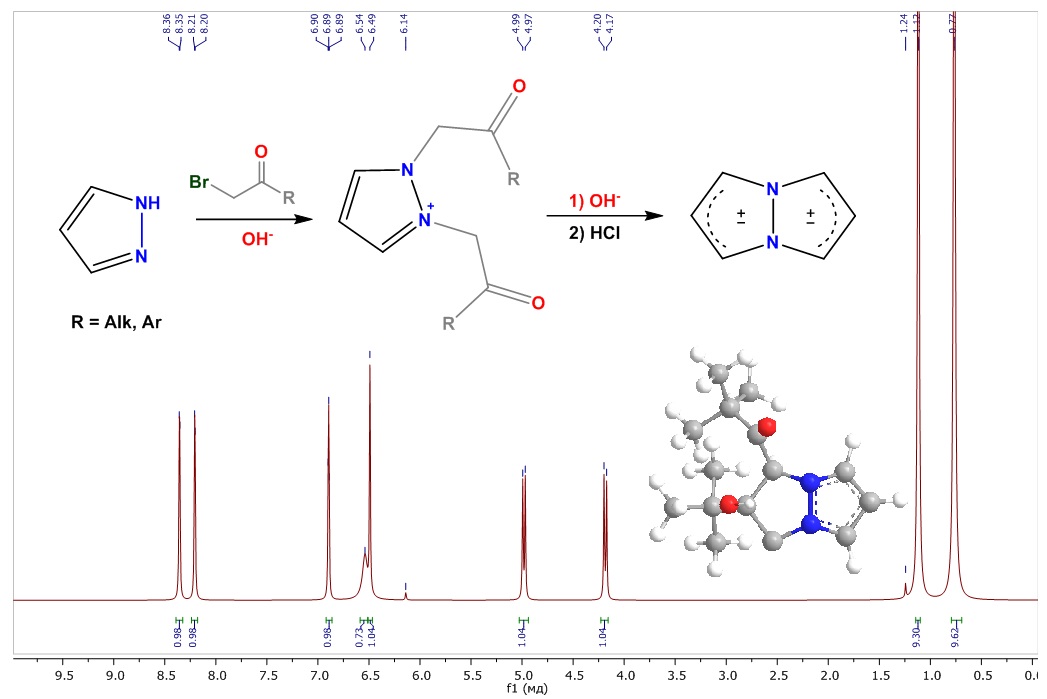
Recently discovered 1,3a,6a-triazapentalene systems are among the new fluorophores whose compact and biocompatible structures are suitable for a variety of biological applications. The wavelengths of 1,3a,6a-triazapentalenes vary depending on the nature of the substituents, allowing for the easy synthesis of yellow and red fluorescent reagents for labeling biomolecules. The prototype of the 1,3a,6a-triazapentalene system (without one aza group) is 3a,6a-diazapentalene, which, originally called pyrazolo[1,2-a]pyrazole, which may also exhibit fluorescent activity. However, their research has been limited to a few papers reporting simple and universal principles for synthesizing the 3a,6a-diazapentalene system, one of which involves double alkylation of pyrazole with α-halocarbonyl compounds and treatment of the resulting products with a base. In this work, all stages of the previously performed synthesis of 1-benzoyl-2-phenyl-3a,6a-diazapentalene by the reaction of N-acylalkylation of pyrazole with α-bromoketones through the stages of formation of pyrazolium cation salts are investigated. Based on the studied data, the studied synthesis conditions were first applied by us in stepwise reactions leading to the possible formation of a tert-butyl derivative of 3a,6a-diazapentalene. As a result of the studies, a new, previously undescribed adduct of 3a,6a-diazapentalene, a bicyclic aldol, was obtained. The structure of the substances of the stepwise synthesis was characterized by NMR, IR spectroscopy and mass spectrometry. The mass fragmentation of intermediate N-alkylacylpyrazoles was also considered in detail.
References
Babaev, E.V., Panshina, S.Yu., Alzhapparova, N.A., Ibraev, M.K., & Usenova, M.S. (2025). 3a,6a-Diazapentaleny (pirazo-lo[1,2-a]pirazoly) [3a,6a-Diazapentalenes (pyrazolo[1,2-a]pyrazoles)]. Izvestiya Akademii nauk. Seriya khimicheskaya — Chemical Bulletin, 74(7), 1958‒1975. [in Russian]. https://doi.org/10.1007/s11172-025-4681-8 DOI: https://doi.org/10.1007/s11172-025-4681-8
Koga, H., Hirobe, M. & Okamoto T. (1978). Mesionic 1,3a,6a-triazapentalenes. Tetrahedron Lett., 19(15), 1291–1294. https://doi.org/10.1016/0040-4039(78)80109-9 DOI: https://doi.org/10.1016/0040-4039(78)80109-9
Namba, K., Osawa, A., Nakayama, A., Mera, A., Tano, F., Chuman, Y., Sakuda, E., Taketsugu T., Sakaguchi, K., Kitamura, N., Tanino, K. (2015). Synthesis of yellow and red fluorescent 1,3a,6a-triazapentalenes and the theoretical investigation of their optical properties. Chemical Science, 6(2), 1083–1093. https://doi.org/10.1039/c4sc02780a DOI: https://doi.org/10.1039/C4SC02780A
Tsuji, D., Nakayama, A., Yamamoto, R., Nagano, S., Taniguchi, T., Sato, R., Karanjit, S., Muguruma, N., Takayama, T., It-oh, K. & Namba K. (2023). 1,3a,6a-Triazapentalene derivatives as photo-induced cytotoxic small fluorescent dyes. Communica-tions Chemistry, 6(1), 37. https://doi.org/10.1038/s42004-023-00838-0 DOI: https://doi.org/10.1038/s42004-023-00838-0
Chen, Y., Wang, D., Petersen, J.L., Akhmedov N.G. & Shi, X. (2010). Synthesis and characterization of organogold com-plexes containing an acid stable Au–C bond through triazole-yne 5-endo-dig cyclization. Chemical Communications, 46, 6147–6149. https://doi.org/10.1039/C0CC01338B DOI: https://doi.org/10.1039/c0cc01338b
Cai, R., Wang, D., Chen, Y., Yan, W., Geise, N.R., Sharma, S., Li, H., Petersen, J.L., Li, M. & Shi., X. (2014). Facile syn-thesis of fluorescent active triazapentalenes through gold-catalyzed triazole–alkyne cyclization. Chemical Communications, 50, 7303–7305. https://doi.org/10.1039/C4CC03175J DOI: https://doi.org/10.1039/C4CC03175J
Wang, Y., Opsomer, T., & Dehaen, W. (2022). Developments in the chemistry of 1,3a,6a-triazapentalenes and their fused analogs. Advances in Heterocyclic Chemistry, 137, 25–70. https://doi.org/10.1016/bs.aihch.2021.10.002 DOI: https://doi.org/10.1016/bs.aihch.2021.10.002
Verbelen, B., Dehaen, W. (2016). Two-Step Synthesis of Fluorescent 3-Arylated 1,3a,6a-Triazapentalenes via a Three-Component Triazolization Reaction. Organic Letters, 18(24), 6412‒6415. https://doi.org/10.1021/acs.orglett.6b03309 DOI: https://doi.org/10.1021/acs.orglett.6b03309
Nakayama, A., Otani, A., Inokuma, T., Tsuji, D., Mukaiyama, H., Nakayama A, Itoh, K., Otaka, A., Tanino, K., & Nam-ba, K. (2020). Development of a 1,3a,6a-triazapentalene derivative as a compact and thiol-specific fluorescent labeling reagent. Communications Chemistry, 3(1), 6, 1–9. https://doi.org/10.1038/s42004-019-0250-0 DOI: https://doi.org/10.1038/s42004-019-0250-0
Ito, M., Mera, A., Mashimo, T., Seki, T., Karanjit, S., Ohashi, E., Nakayama, A., Kitamura, K., Hamura, T., Ito, H., & Nam-ba, K. (2018). Synthesis and Evaluation of a 1,3a,6a-Triazapentalene (TAP)-Bonded System. Chemistry — A European Journal, 24(67), 17727‒17733. https://doi.org/10.1002/chem.201804733 DOI: https://doi.org/10.1002/chem.201804733
Legentil, P., Chadeyron, G., Therias, S., Chopin, N., Sirbu, D., Suzenet, F., & Leroux, F. (2020). Luminescent N-hetero-cycles based molecular backbone interleaved within LDH host structure and dispersed into polymer. Applied Clay Science, 189, 105561. https://doi.org/10.1016/j.clay.2020.105561 DOI: https://doi.org/10.1016/j.clay.2020.105561
Wang, Y, Opsomer T, Van Meervelt, L., & Dehaen, W. (2020) Ring-Degenerate Rearrangement Resulting from the Azo Coupling Reaction of a 3-Aryl-1,3a,6a-triazapentalene. The Journal of Organic Chemistry, 85(14), 9434‒9439. https://doi.org/10.1021/acs.joc.0c01153 DOI: https://doi.org/10.1021/acs.joc.0c01153
Sirbu, D., Diharce, J., Martinić, I., Chopin, N., Eliseeva, S.V., Guillaumet, G., Petoud, S., Bonnet, P., & Suzenet, F. (2019) An original class of small sized molecules as versatile fluorescent probes for cellular imaging. Chemical Communications, 55(54), 7776‒7779. https://doi.org/10.1039/c9cc03765a DOI: https://doi.org/10.1039/C9CC03765A
Wang, Y., Pham, T. C., Huang, J., Wu, J., & Dehaen, W. (2025). Heteroaryl-Fused Triazapentalenes: Synthesis and Aggre-gation-Induced Emission. Molecules, 30(1), 156. https://doi.org/10.3390/molecules30010156 DOI: https://doi.org/10.3390/molecules30010156
Wang, Y., Opsomer, T., de Jong, F., Verhaeghe, D., Mulier, M., Van Meervelt, L., Van der Auweraer, M., & Dehaen, W. (2024). Palladium-Catalyzed Arylations towards 3,6-Diaryl-1,3a,6a-triazapentalenes and Evaluation of Their Fluorescence Proper-ties. Molecules, 29(10), 2229. https://doi.org/10.3390/molecules29102229 DOI: https://doi.org/10.3390/molecules29102229
Solomons, T.W.G., & Voigt, C.F. (1966). The Diazapentalene System. IV. The Parent Pyrazolo[1,2-α]pyrazole and Deriva-tives. Journal of the American Chemical Society, 88(1), 1992–1994. https://doi.org/10.1021/ja00961a025 DOI: https://doi.org/10.1021/ja00961a025
Solomons, T.W.G., Fowler, F.W., & Calderazzo, J. (1965). The Diazapentalene System. 1-Benzoyl-2-phenylpyrazolo[1,2-a]pyrazole Derivatives. Journal of the American Chemical Society, 87(3), 528‒531. https://doi.org/10.1021/ja01081a023 DOI: https://doi.org/10.1021/ja01081a023
Solomons, T.W.G., & Voigt, C.F. (1965). 4,8-Diazapentalene. Journal of the American Chemical Society, 87(22), 5256. https://doi.org/10.1021/ja00950a052 DOI: https://doi.org/10.1021/ja00950a052
Trofimenko, S. (1966). 3a,6a-Diazapentalenes. Synthesis and Chemistry. Journal of the American Chemical Society, 88(23), 5588‒5592. https://doi.org/10.1021/ja00975a043 DOI: https://doi.org/10.1021/ja00975a043
Trofimenko, S. (1965). 3a,6a-Diazapentalene (Pyrazolo[1,2-a]pyrazole). Journal of the American Chemical Society, 87(19), 4393‒4394. https://doi.org/10.1021/ja00947a038 DOI: https://doi.org/10.1021/ja00947a038
Ramsden, C.A. (1977). Mesomeric betaine derivatives of heteropentalenes. Tetrahedron, 33(24) 3193‒3202. https://doi.org/10.1016/0040-4020(77)80141-5 DOI: https://doi.org/10.1016/0040-4020(77)80141-5
Kawase, M., Sakagami, H., Motohashi, N. (2007). The Chemistry of Bioactive Mesoionic Heterocycles. In: Motohashi, N. (eds) Bioactive Heterocycles VII. Topics in Heterocyclic Chemistry, 16. Berlin, Springer, (рр. 135–152). https://doi.org/ 10.1007/7081_2007_096 DOI: https://doi.org/10.1007/7081_2007_096
Sizov, G.N., & Babaev, E.V. (2023). Criteria for a Structure to be Mesoionic. Match, 89(1), 5‒47. https://doi.org/ 10.46793/match.89-1.005S DOI: https://doi.org/10.46793/match.89-1.005S
Alkorta, I., Blanco, F., & Elguero, J. (2009). Theoretical studies of azapentalenes. Part 4: Theoretical study of the properties of 3a,6a-diazapentalene. Tetrahedron, 6(29‒30), 5760‒5766. https://doi.org/10.1016/j.tet.2009.05.017 DOI: https://doi.org/10.1016/j.tet.2009.05.017
Rague, S.P., Маеrкеr, C., Dransfeld, A., Jiao, H., & Eikema Hommes, N.J.R. (1996). Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. Journal of the American Chemical Society, 118(26), 6317‒6318. https://doi.org/ 10.1021/ja960582d DOI: https://doi.org/10.1021/ja960582d
Puello, J.Q., Obando, B.I., Foces-Foces, C., Infantes, L., Claramunt, R.M., Cabildo, P., Jimenez, J.A., & Elguero, J. (1997). Structure and Tautomerism of 3(5)-Amino-5(3)-arylpyrazoles in the Solid State and in Solution: an X-Ray and NMR Study. Tetra-hedron, 53(31), 10783‒10802. https://doi.org/10.1016/s0040-4020(97)00678-9 DOI: https://doi.org/10.1016/S0040-4020(97)00678-9
Stanovnik, B., & Svete, J. (2003). Product Class 1: Pyrazoles. ChemInform, 34(46), 15‒225 https://doi.org/10.1002/ chin.200346258 DOI: https://doi.org/10.1002/chin.200346258
La Cour, T., Rasmussen, S.E., Hopf, H., Waisvisz, J.M., van der Hoeven, M.G., & Swahn, C. -G. (1973). The Structure of Pyrazole, C3H4N2, at 295 K and 108 K as determined by X-Ray Diffraction. Acta Chemica Scandinavica, 27, 1845‒1854. https://doi.org/10.3891/acta.chem.scand.27-1845 DOI: https://doi.org/10.3891/acta.chem.scand.27-1845
Sikora, M., & Katrusiak, A. (2013). Pressure-Controlled Neutral–Ionic Transition and Disordering of NH···N Hydrogen Bonds in Pyrazole. The Journal of Physical Chemistry C, 117(20), 10661‒10668. https://doi.org/10.1021/jp401389v DOI: https://doi.org/10.1021/jp401389v
Millán, J.C., & Portilla, J. (2019). Recent advances in the synthesis of new pyrazole derivatives. Italian Society of Chemis-try, 194‒223. https://doi.org/10.17374/targets.2019.22.194
Perrin, D.D. (1965). Dissociation Constants of Organic Bases in Aqueous Solution. (Supplement, 1972). London: Butterworths
Smith, M.B., & March, J. (2001). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Molecules, 6(12), 1064‒1065. https://doi.org/10.3390/61201064 DOI: https://doi.org/10.3390/61201064
Rybakov, V. B., Shchetinin, A. V., Babaev, E. V., Panshina, S. Yu., Alzhaparova, N. A., & Ibraev, M. K. (2024). CCDC 2393860: Experimental crystal structure determination. CSD Communication. https://doi.org/10.5517/ccdc.csd.cc2lc099
Soignet, D. M., Boudreaux, G. J., Berni, R. J., & Gonzales, E. J. (1970). Nuclear Magnetic Resonance Studies of Substituted Cyclic Ureas. Applied Spectroscopy, 24(2), 272–276. https://doi.org/10.1366/000370270774371985 DOI: https://doi.org/10.1366/000370270774371985
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nazym A. Alzhapparova, Svetlana Yu. Panshina, Marat K. Ibrayev, Eugene V. Babaev

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



