Synthesis, Characterization and Computational Study of Novel Copper(II) Chelate Complexes Ligated by Pyridyl-Containing Beta-Diketonates
DOI:
https://doi.org/10.31489/2959-0663/2-25-5Keywords:
complexation, copper, diketone, atomic emission spectroscopy, mass spectrometry, IR spectroscopy, chelate, complex compoundAbstract
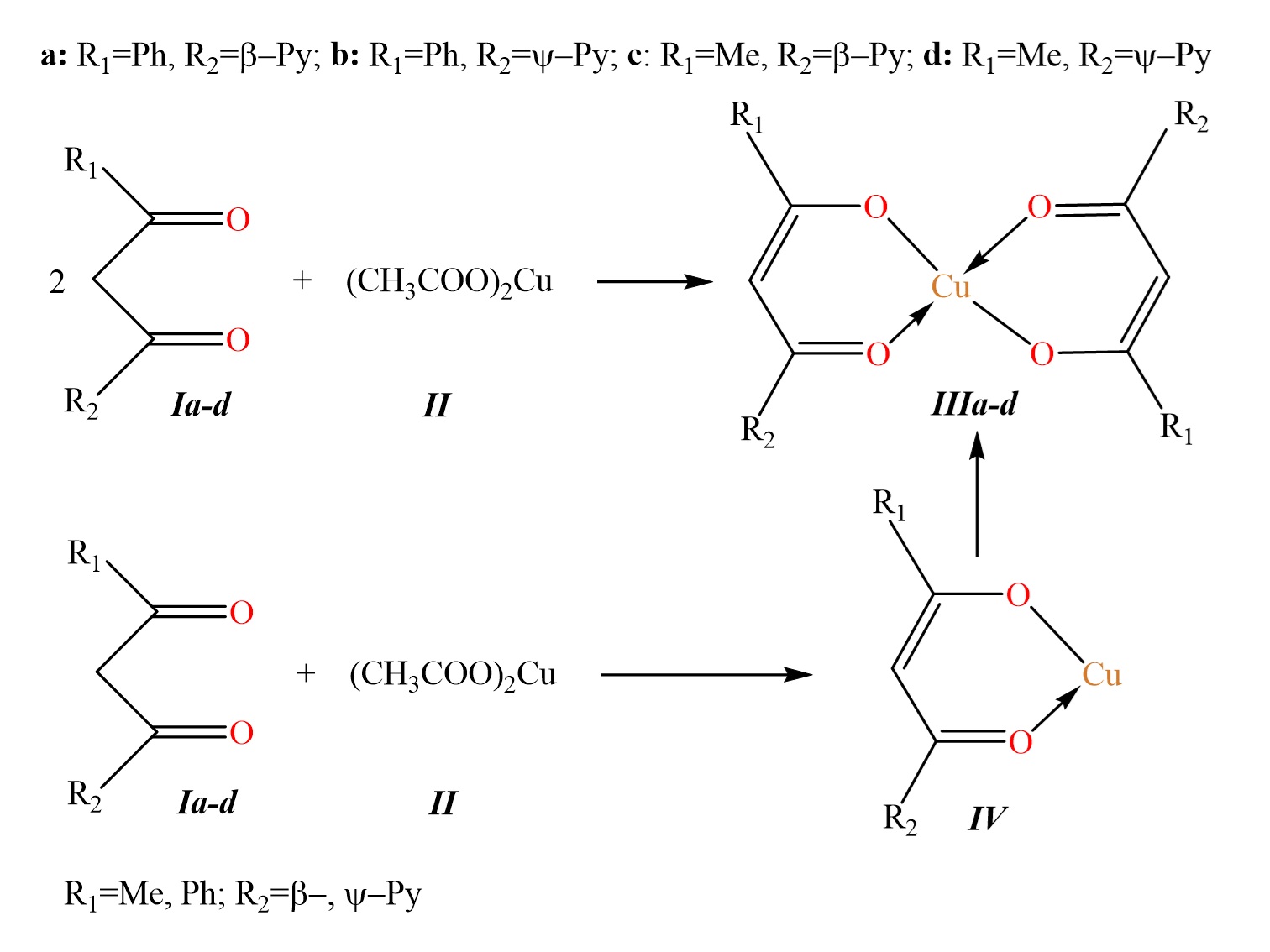
Chelate complexes of copper(II) are widely used today in various sectors of the national economy, including medicine and pharmacology, biotechnology and agriculture, catalysis, and materials science. Pyridyl-containing β-diketones possess unique properties and can act as chelating ligands for metals, making them promising candidates for the development of metal-based pharmaceutical compounds. Therefore, the development and discovery of new copper(II) chelate complexes are of great interest and practical significance. In this work, new copper(II) chelate complexes of pyridyl-containing beta-diketonates were synthesized for the first time. Complexation between pyridyl-containing β-diketones and copper(II) acetate with molar ratio 2:1 was carried out in ethanol at a temperature not exceeding 50 ºC for 1 hour, with the yield of products IIIa–d ranging from 8.5 % to 31.3 %. The synthesized complexes were characterized using IR spectroscopy, atomic emission spectroscopy, and mass spectrometry, as well as DFT calculations, PASS prediction, and molecular docking. It was shown that all synthesized chelates exhibit biological activity as nicotinic receptor antagonists, with Pa values ranging from 0.821 to 0.915, and as dehydro-L-gulonate decarboxylase inhibitors, with Pa values exceeding 0.75. Molecular docking simulations with the alpha2 nicotinic acetylcholine receptor (PDB ID: 5FJV) confirmed high potential of synthesized chelates as nicotinic receptor antagonists and they can be recommended for further evaluation of therapeutic relevance through in vitro and in vivo studies.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Alexey A. Kukushkin, Elizaveta V. Kudashova, Evgeny V. Root, Anna S. Kositsyna, Ilya S. Ponomarev, Alexey V. Lyubyashkin, Irina A. Pustolaikina

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



