A Series of Novel Integrastatins Analogues: In silico Study of Physicochemical and Bioactivity Parameters
DOI:
https://doi.org/10.31489/2959-0663/4-24-13Keywords:
integrastatins, in silico, molecular docking, computational study, biological activity, ADMET, quantum chemical calculations, DFT, B3LYP, antiviral activity, global chemical reactivity descriptors, HIV-1, SARS-CoV2Abstract
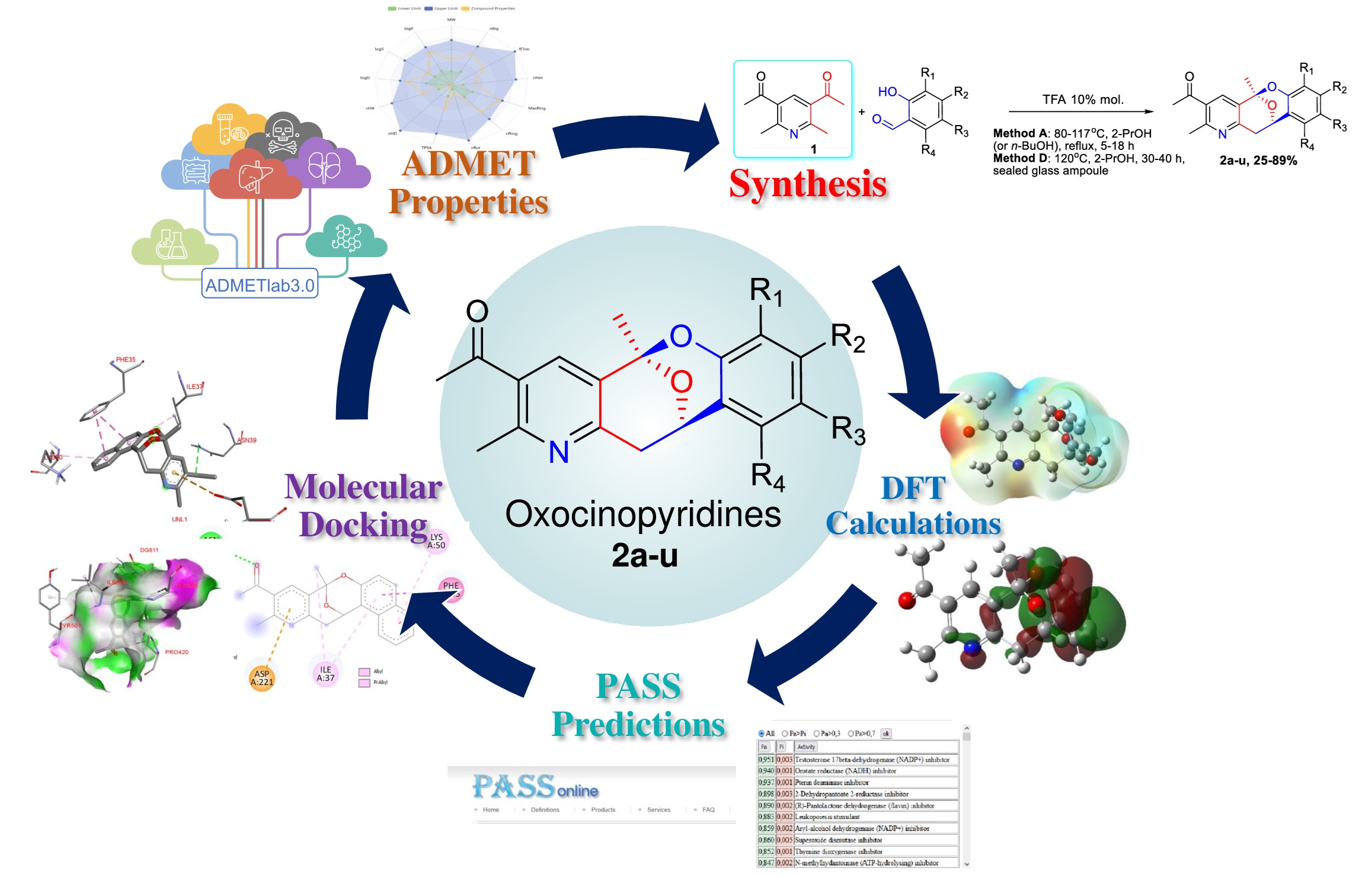
Integrastatins are naturally occurring heterotetracyclic compounds with a broad spectrum of biological activity. A number of new structural analogues of integrastatins 2a-u have been synthesized using a novel one-step method [1], but a systematic theoretical study of their structural features and biological activity has not been realized. This study aimed to in silico investigate physicochemical and bioactivity properties for a series of 21 new synthetic analogues of natural integrastatins. Global chemical reactivity descriptors was assessed using DFT B3LYP 6-311++G(d, p) CPCM (solvent — water) calculations. High ionization potential IP in the range from 5.9 to 7.1 eV and electron affinity EA at the level of 2.1 to 3.2 eV were shown, which together with a sufficiently large energy gap DEgap from 3.8 to 4.6 eV indicates the hard nature of the compounds 2a-u. Antiviral activity and inhibitory potential as CYP2C19 inducers were identified using the PASSOnline resource. According to the results of molecular docking studies Human immunodeficiency virus HIV-1 reverse transcriptase protein (PDB ID: 3V81) and protein of the RNA-dependent RNA polymerase of the SARS-CoV-2 (PDB ID: 7AAP) can serve as a likely biological target for the compounds 2a-u. Potentially high oral efficacy and a promising safety profile for the therapeutic use were showed using ADMETlab 3.0 online portal. Further experimental in vitro and in vivo studies of the pharmaceutical potential of compounds 2a-u is need for more accurate evaluation the assumptions made on the basis of in silico approach.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Aida S. Rakhimzhanova, Ruslan A. Muzaparov, Irina A. Pustolaikina, Alfiya F. Kurmanova , Sergey N. Nikolskiy, Darya D. Kapishnikova, Alena L. Stalinskaya, Ivan V. Kulakov

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



