Transport Properties of Cation-Exchange Membranes Obtained by Pore Filling of Track-Etched Membranes with Perfluorosulfonic Acid Polymer
DOI:
https://doi.org/10.31489/2959-0663/3-25-2Keywords:
cation-exchange membrane, track-etched membrane, pore filling membrane, perfluorosulfonic, electrical conductivity, osmotic flux, diffusion permeability, true transport numbersAbstract
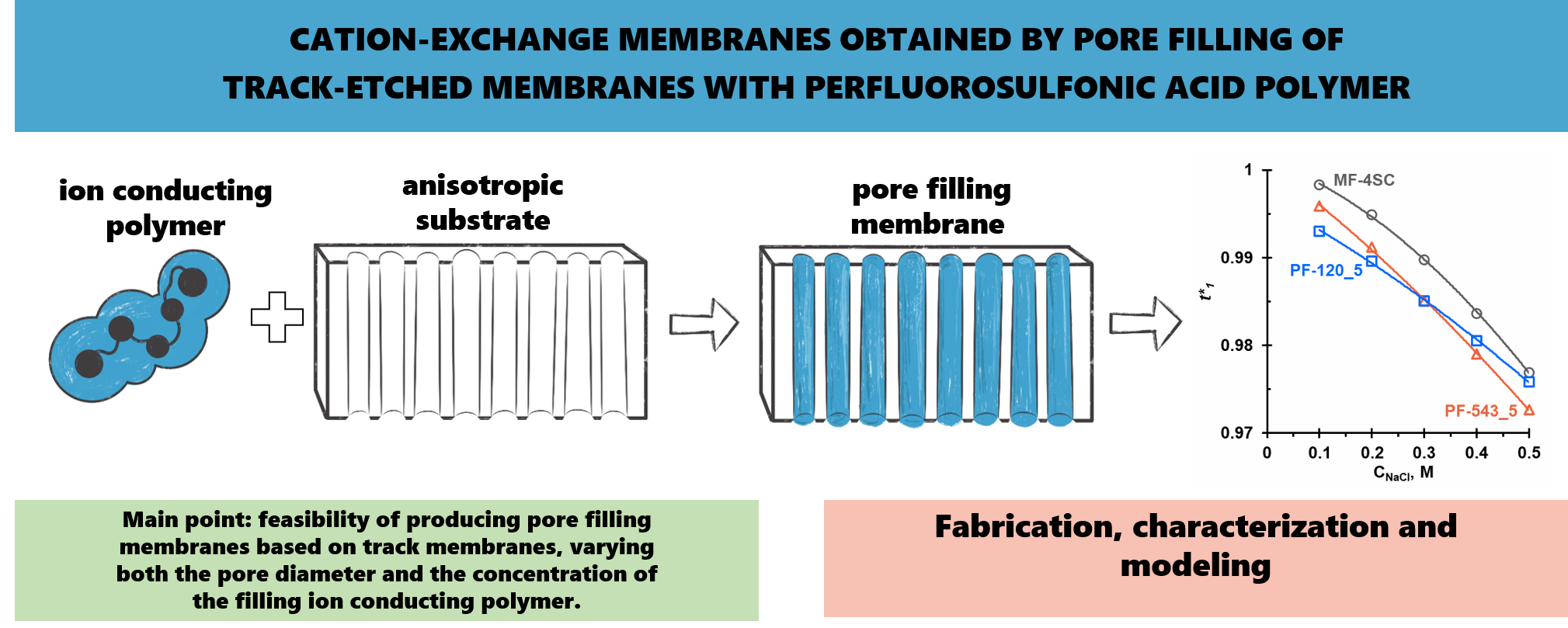
In this paper the correlation between the structural characteristics and transport properties of pore-filling membranes formed by embedding an ion-conducting polymer into track-etched substrates was studied. Cation-exchange membranes were fabricated by filling the pores of track-etched membranes with a perfluorosulfonic acid polymer (trade mark LF-4SC). The resulting membranes differed in the volume fraction of the ion-conducting polymer and in the presence or absence of a surface LF-4SC layer. SEM and ATR-FTIR spectroscopy were employed to characterize the chemical composition and structure of the membranes. A comparative analysis of ion-exchange capacity and water uptake was carried out. Concentration dependences of specific electrical conductivity and diffusion permeability in NaCl solutions were obtained. The effects of pore filling degree and LF-4SC layer thickness on osmotic transport, diffusion permeability, and selectivity were analyzed. The electrical resistance of the prepared membranes was found to be comparable to that of the commercial MF-4SC membrane, produced from the same perfluorosulfonic acid polymer, despite a significant fraction of the polymer in the new membranes not participating in counterion transport. The observed structure–property relationships were interpreted within the framework of the two-phase microheterogeneous model, providing insight into the functional behavior of the composite membranes.
References
Nikonenko, V., Nebavsky, A., Mareev, S., Kovalenko, A., Urtenov, M., & Pourcelly, G. (2018). Modelling of ion transport in electromembrane systems: impacts of membrane bulk and surface heterogeneity. Applied Sciences, 9(1), 25. https://doi.org/10.3390/app9010025 DOI: https://doi.org/10.3390/app9010025
Stenina, I., Golubenko, D., Nikonenko, V., & Yaroslavtsev, A. (2020). Selectivity of transport processes in ion-exchange membranes: relationship with the structure and methods for its improvement. International Journal of Molecular Sciences, 21(15), 5517. https://doi.org/10.3390/ijms21155517 DOI: https://doi.org/10.3390/ijms21155517
Gnusin, N. P., Berezina, N. P., Kononenko, N. A., & Dyomina, O. A. (2004). Transport structural parameters to characterize ion exchange membranes. Journal of Membrane Science, 243(1–2), 301–310. https://doi.org/10.1016/j.memsci.2004.06.033 DOI: https://doi.org/10.1016/j.memsci.2004.06.033
Zabolotsky, V. I., & Nikonenko, V. V. (1993). Effect of structural membrane inhomogeneity on transport properties. Jour-nal of Membrane Science, 79(2–3), 181–198. https://doi.org/10.1016/0376-7388(93)85115-D DOI: https://doi.org/10.1016/0376-7388(93)85115-D
Nichka, V. S., Mareev, S. A., Porozhnyy, M. V., Shkirskaya, S. A., Safronova, E. Yu., Pismenskaya, N. D., & Nikonenko, V. V. (2019). Modified microheterogeneous model for describing electrical conductivity of membranes in dilute electrolyte solutions. Membranes and Membrane Technologies, 1(3), 190–199. https://doi.org/10.1134/S2517751619030028 DOI: https://doi.org/10.1134/S2517751619030028
Nichka, V. S., Mareev, S. A., Apel, P. Yu., Sabbatovskiy, K. G., Sobolev, V. D., & Nikonenko, V. V. (2022). Modeling the conductivity and diffusion permeability of a track-etched membrane taking into account a loose layer. Membranes, 12(12), 1283. https://doi.org/10.3390/membranes12121283 DOI: https://doi.org/10.3390/membranes12121283
Porozhnyy, M., Huguet, P., Cretin, M., Safronova, E., & Nikonenko, V. (2016). Mathematical modeling of transport proper-ties of proton-exchange membranes containing immobilized nanoparticles. International Journal of Hydrogen Energy, 41(34), 15605–15614. https://doi.org/10.1016/j.ijhydene.2016.06.057 DOI: https://doi.org/10.1016/j.ijhydene.2016.06.057
Choy, T. C. (2015). Effective Medium Theory: Principles and Applications. Oxford University Press. DOI: https://doi.org/10.1093/acprof:oso/9780198705093.001.0001
Sedkaoui, Y., Szymczyk, A., Lounici, H., & Arous, O. (2016). A new lateral method for characterizing the electrical con-ductivity of ion-exchange membranes. Journal of Membrane Science, 507, 34–42. https://doi.org/10.1016/j.memsci.2016.02.003 DOI: https://doi.org/10.1016/j.memsci.2016.02.003
Vyas, P. V, Ray, P., Adhikary, S. K., Shah, B. G., & Rangarajan, R. (2003). Studies of the effect of variation of blend ratio on permselectivity and heterogeneity of ion-exchange membranes. Journal of Colloid and Interface Science, 257, 127–134. https://doi.org/10.1016/S0021-9797(02)00025-5 DOI: https://doi.org/10.1016/S0021-9797(02)00025-5
Davydov, D., Nosova, E., Loza, S., Achoh, A., Korzhov, A., Sharafan, M., & Melnikov, S. (2021). Use of the microhetero-geneous model to assess the applicability of ion-exchange membranes in the process of generating electricity from a concentration gradient. Membranes, 11(6), 406. https://doi.org/10.3390/membranes11060406 DOI: https://doi.org/10.3390/membranes11060406
Gohil, G. (2004). Comparative studies on electrochemical characterization of homogeneous type of ion-exchange mem-branes. Journal of Membrane Science, 240(1–2), 211–219. https://doi.org/10.1016/j.memsci.2004.04.022 DOI: https://doi.org/10.1016/j.memsci.2004.04.022
Le, X. T., Bui, T. H., Viel, P., Berthelot, T., & Palacin, S. (2009). On the structure–properties relationship of the AMV anion exchange membrane. Journal of Membrane Science, 340(1–2), 133–140. https://doi.org/10.1016/j.memsci.2009.05.025 DOI: https://doi.org/10.1016/j.memsci.2009.05.025
Loza, N. V, & Kutenko, N. A. (2024). Effect of Nature and Charge of Counterions and Co-Ions on Electrotransport Proper-ties of Heterogeneous Anion Exchange Membranes. Membranes and Membrane Technologies, 6(3), 193–204. https://doi.org/10.1134/S2517751624600274 DOI: https://doi.org/10.1134/S2517751624600274
Novikova, S. A., Volodina, E. I., Pis’menskaya, N. D., Veresov, A. G., Stenina, I. A., & Yaroslavtsev, A. B. (2005). Ionic transport in cation-exchange membranes MK-40 modified with zirconium phosphate. Russian Journal of Electrochemistry, 41(10), 1070–1076. https://doi.org/10.1007/s11175-005-0183-z DOI: https://doi.org/10.1007/s11175-005-0183-z
Zabolotskii, V. I., Loza, S. A., & Sharafan, M. V. (2005). Physicochemical properties of profiled heterogeneous ion-exchange membranes. Russian Journal of Electrochemistry, 41(10), 1053–1060. https://doi.org/10.1007/s11175-005-0180-2 DOI: https://doi.org/10.1007/s11175-005-0180-2
Tuan, L. X., Verbanck, M., Buess-Herman, C., & Hurwitz, H. D. (2006). Properties of CMV cation exchange membranes in sulfuric acid media. Journal of Membrane Science, 284(1–2), 67–78. https://doi.org/10.1016/j.memsci.2006.06.036 DOI: https://doi.org/10.1016/j.memsci.2006.06.036
Iddya, A., Zarzycki, P., Kingsbury, R., Khor, C. M., Ma, S., Wang, J., Wheeldon, I., Ren, Z. J., Hoek, E. M. V., & Jassby, D. (2022). A reverse-selective ion exchange membrane for the selective transport of phosphates via an outer-sphere complexation–diffusion pathway. Nature Nanotechnology, 17(11), 1222–1228. https://doi.org/10.1038/s41565-022-01209-x DOI: https://doi.org/10.1038/s41565-022-01209-x
Pismenskaya, N. D., Nevakshenova, E. E., & Nikonenko, V. V. (2018). Using a single set of structural and kinetic parame-ters of the microheterogeneous model to describe the sorption and kinetic properties of ion-exchange membranes. Petroleum Chem-istry, 58(6), 465–473. https://doi.org/10.1134/S0965544118060087 DOI: https://doi.org/10.1134/S0965544118060087
Salmeron-Sanchez, I., Asenjo-Pascual, J., Avilés-Moreno, J. R., & Ocón, P. (2022). Microstructural description of ion ex-change membranes: The effect of PPy-based modification. Journal of Membrane Science, 659, 120771. https://doi.org/10.1016/j.memsci.2022.120771 DOI: https://doi.org/10.1016/j.memsci.2022.120771
Berezina, N. P., Kononenko, N. A., Dyomina, O. A., & Gnusin, N. P. (2008). Characterization of ion-exchange membrane materials: Properties vs structure. Advances in Colloid and Interface Science, 139(1–2), 3–28. https://doi.org/10.1016/j.cis.2008.01.002 DOI: https://doi.org/10.1016/j.cis.2008.01.002
Xu, T., & Wang, Y. (2024). Ion Exchange Membranes: Design, Preparation, and Applications. Wiley-VCH GmbH. DOI: https://doi.org/10.1002/9783527841448
Stránská, E., & Neděla, D. (2018). Reinforcing fabrics as the mechanical support of ion exchange membranes. Journal of Industrial Textiles, 48(2), 432–447. https://doi.org/10.1177/1528083717732075 DOI: https://doi.org/10.1177/1528083717732075
Sarapulova, V., Pismenskaya, N., Titorova, V., Sharafan, M., Wang, Y., Xu, T., Zhang, Y., & Nikonenko, V. (2021). Transport characteristics of CJMAEDTM homogeneous anion exchange membranes in sodium chloride and sodium sulfate solutions. International Journal of Molecular Sciences, 22(3), 1415. https://doi.org/10.3390/ijms22031415 DOI: https://doi.org/10.3390/ijms22031415
Eti, M., Hidayati Othman, N., Guuml; ler, E., & Kabay, N. (2021). Ion exchange membranes for reverse electrodialysis RED) applications—Recent developments. Journal of Membrane Science and Research, 7(4). https://doi.org/10.22079/jmsr.2021.534937.1482
Niccolai, F., Guazzelli, E., El Koura, Z., Pucher, I., & Martinelli, E. (2025). A critical update on the design of dense ion‐conducting membranes for redox flow batteries. Advanced Sustainable Systems, 9(2), 2400661. https://doi.org/10.1002/adsu.202400661 DOI: https://doi.org/10.1002/adsu.202400661
Yang, S., Choi, Y. -W., Choi, J., Jeong, N., Kim, H., Jeong, H., Byeon, S. Y., Yoon, H., & Kim, Y. H. (2019). Green fabrica-tion of pore-filling anion exchange membranes using R2R processing. Journal of Membrane Science, 584, 181–190. https://doi.org/10.1016/j.memsci.2019.04.075 DOI: https://doi.org/10.1016/j.memsci.2019.04.075
Gloukhovski, R., Freger, V., & Tsur, Y. (2018). Understanding methods of preparation and characterization of pore-filling polymer composites for proton exchange membranes: A beginner’s guide. Reviews in Chemical Engineering, 34(4), 455–479. https://doi.org/10.1515/revce-2016-0065 DOI: https://doi.org/10.1515/revce-2016-0065
Fan, H., Xu, Y., Zhao, F., Chen, Q. -B., Wang, D., & Wang, J. (2023). A novel porous asymmetric cation exchange mem-brane with thin selective layer for efficient electrodialysis desalination. Chemical Engineering Journal, 472, 144856. https://doi.org/10.1016/j.cej.2023.144856
Wu, J., Dai, Q., Zhang, H., & Li, X. (2020). Recent development in composite membranes for flow batteries. ChemSus-Chem, 13(15), 3805–3819. https://doi.org/10.1002/cssc.202000633 DOI: https://doi.org/10.1002/cssc.202000633
Chavan, V., Agarwal, C., Adya, V. C., & Pandey, A. K. (2018). Hybrid organic-inorganic anion-exchange pore-filled mem-branes for the recovery of nitric acid from highly acidic aqueous waste streams. Water Research, 133, 87–98. https://doi.org/10.1016/j.watres.2018.01.023 DOI: https://doi.org/10.1016/j.watres.2018.01.023
Kim, D.-H., Seo, S.-J., Lee, M.-J., Park, J.-S., Moon, S.-H., Kang, Y. S., Choi, Y.-W., & Kang, M.-S. (2014). Pore-filled ani-on-exchange membranes for non-aqueous redox flow batteries with dual-metal-complex redox shuttles. Journal of Membrane Sci-ence, 454, 44–50. https://doi.org/10.1016/j.memsci.2013.11.051 DOI: https://doi.org/10.1016/j.memsci.2013.11.051
Shi, S., Weber, A. Z., & Kusoglu, A. (2016). Structure/property relationship of Nafion XL composite membranes. Journal of Membrane Science, 516, 123–134. https://doi.org/10.1016/j.memsci.2016.06.004 DOI: https://doi.org/10.1016/j.memsci.2016.06.004
Dalal, U., Kapoor, M., & Verma, A. (2023). Low-cost pore-filled PVDF–Nafion composite membrane for the vanadium re-dox flow battery. Energy & Fuels, 37(17), 13457–13466. https://doi.org/10.1021/acs.energyfuels.3c01932 DOI: https://doi.org/10.1021/acs.energyfuels.3c01932
Fang, Y., & Leddy, J. (1995). Surface diffusion in microstructured, ion-exchange matrixes: Nafion/Neutron Track-Etched Polycarbonate Membrane composites. The Journal of Physical Chemistry, 99(16), 6064–6073. https://doi.org/10.1021/j100016a049 DOI: https://doi.org/10.1021/j100016a049
Kang, S. E., & Lee, C. H. (2015). Perfluorinated sulfonic acid ionomer-PTFE pore-filling membranes for polymer electro-lyte membrane fuel cells. Membrane Journal, 25(2), 171–179. https://doi.org/10.14579/MEMBRANE_JOURNAL.2015.25.2.171 DOI: https://doi.org/10.14579/MEMBRANE_JOURNAL.2015.25.2.171
Cha, J.-E., Seo, M. H., Choi, Y.-W., & Kim, W. B. (2021). A practical approach to measuring the ion-transport number of cation-exchange membranes: Effects of junction potential and analyte concentration. Journal of Membrane Science, 635, 119471. https://doi.org/10.1016/j.memsci.2021.119471 DOI: https://doi.org/10.1016/j.memsci.2021.119471
Yang, S., Choi, Y.-W., Choi, J., Jeong, N., Kim, H., Nam, J.-Y., & Jeong, H. (2019). R2R fabrication of pore-filling cation-exchange membranes via one-time impregnation and their application in reverse electrodialysis. ACS Sustainable Chemistry & Engineering, acssuschemeng.9b01450. https://doi.org/10.1021/acssuschemeng.9b01450 DOI: https://doi.org/10.1021/acssuschemeng.9b01450
Wang, B., Yan, J., Wang, H., Li, R., Fu, R., Jiang, C., Nikonenko, V., Pismenskaya, N., Wang, Y., & Xu, T. (2024). Solvent-free fabrication of pore-filling cation-exchange membranes for highly efficient desalination. Chemical Engineering Science, 287, 119782. https://doi.org/10.1016/j.ces.2024.119782 DOI: https://doi.org/10.1016/j.ces.2024.119782
Wang, M., An, Q.-F., Wu, L.-G., Mo, J.-X., & Gao, C.-J. (2007). Preparation of pH-responsive phenolphthalein poly(ether sulfone) membrane by redox-graft pore-filling polymerization technique. Journal of Membrane Science, 287(2), 257–263. https://doi.org/10.1016/j.memsci.2006.10.049 DOI: https://doi.org/10.1016/j.memsci.2006.10.049
Hu, K., & Dickson, J. M. (2007). Development and characterization of poly(vinylidene fluoride)–poly(acrylic acid) pore-filled pH-sensitive membranes. Journal of Membrane Science, 301(1–2), 19–28. https://doi.org/10.1016/j.memsci.2007.05.031 DOI: https://doi.org/10.1016/j.memsci.2007.05.031
Hu, K., & Dickson, J. M. (2008). Modelling of the pore structure variation with pH for pore-filled pH-sensitive poly(vinylidene fluoride)–poly(acrylic acid) membranes. Journal of Membrane Science, 321(2), 162–171. https://doi.org/10.1016/j.memsci.2008.04.046 DOI: https://doi.org/10.1016/j.memsci.2008.04.046
Mika, A. M., Childs, R. F., & Dickson, J. M. (2002). Salt separation and hydrodynamic permeability of a porous membrane filled with pH-sensitive gel. Journal of Membrane Science, 206(1–2), 19–30. https://doi.org/10.1016/S0376-7388(01)00474-4 DOI: https://doi.org/10.1016/S0376-7388(01)00474-4
Xie, R., Chu, L.-Y., Chen, W.-M., Xiao, W., Wang, H.-D., & Qu, J.-B. (2005). Characterization of microstructure of poly(N-isopropylacrylamide)-grafted polycarbonate track-etched membranes prepared by plasma-graft pore-filling polymerization. Journal of Membrane Science, 258(1–2), 157–166. https://doi.org/10.1016/j.memsci.2005.03.012 DOI: https://doi.org/10.1016/j.memsci.2005.03.012
Zou, Z., Wu, L., Luo, T., Yan, Z., & Wang, X. (2021). Assessment of anion exchange membrane selectivity with ionic membrane conductivity, revised with Manning’s theory or the Kohlrausch’s law. Journal of Membrane Science, 635, 119496. https://doi.org/10.1016/j.memsci.2021.119496 DOI: https://doi.org/10.1016/j.memsci.2021.119496
Golubenko, D. V., Yurova, P. A., Desyatov, A. V., Stenina, I. A., Kosarev, S. A., & Yaroslavtsev, A. B. (2022). Pore filled ion-conducting materials based on track-etched membranes and sulfonated polystyrene. Membranes and Membrane Technologies, 4(6), 398–403. https://doi.org/10.1134/S2517751622060026 DOI: https://doi.org/10.1134/S2517751622060026
Gloukhovski, R., Tsur, Y., & Freger, V. (2017). A Nafion‐filled polycarbonate track‐etched composite membrane with en-hanced selectivity for direct methanol fuel cells. Fuel Cells, 17(1), 56–66. https://doi.org/10.1002/fuce.201600154 DOI: https://doi.org/10.1002/fuce.201600154
Parmanbek, N., Sütekin, D. S., Barsbay, M., Mashentseva, A. A., Zheltov, D. A., Aimanova, N. A., Jakupova, Z. Ye., & Zdo-rovets, M. V. (2022). Hybrid PET track-etched membranes grafted by well-defined poly(2-(dimethylamino)ethyl methacrylate) brushes and loaded with silver nanoparticles for the removal of As(III). Polymers, 14(19), 4026. https://doi.org/10.3390/polym14194026 DOI: https://doi.org/10.3390/polym14194026
Korolkov, I. V., Yeszhanov, A. B., Shakayeva, A. Kh., Shlimas, D. I., Zhumazhanova, A., & Zdorovets, M. V. (2022). Pho-to-induced graft (co)polymerization of glycidyl methacrylate and acrylonitrile on PET ion-track membranes for electrochemical detection of uranyl ions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 648, 129086. https://doi.org/10.1016/j.colsurfa.2022.129086 DOI: https://doi.org/10.1016/j.colsurfa.2022.129086
Meyer, N., Arroyo, N., Janot, J. -M., Lepoitevin, M., Stevenson, A., Nemeir, I. A., Perrier, V., Bougard, D., Belondrade, M., Cot, D., Bentin, J., Picaud, F., Torrent, J., & Balme, S. (2021). Detection of amyloid-β fibrils using track-etched nanopores: effect of geometry and crowding. ACS Sensors, 6(10), 3733–3743. https://doi.org/10.1021/acssensors.1c01523 DOI: https://doi.org/10.1021/acssensors.1c01523
Hoek, E. M. V., & Tarabara, V. V. (2013). Encyclopedia of Membrane Science and Technology. Wiley. https://doi.org/10.1002/9781118522318 DOI: https://doi.org/10.1002/9781118522318
Sarapulova, V. V., Pasechnaya, E. L., Titorova, V. D., Pismenskaya, N. D., Apel, P. Yu., & Nikonenko, V. V. (2020). Elec-trochemical properties of ultrafiltration and nanofiltration membranes in solutions of sodium and calcium chloride. Membranes and Membrane Technologies, 2(5), 332–350. https://doi.org/10.1134/S2517751620050066 DOI: https://doi.org/10.1134/S2517751620050066
Yaroslavcev, A. B., & Nikonenko, V. V. (2009). Ionoobmennye membrannye materialy: svojstva, mo-difikaciya i praktich-eskoe primenenie [Ion-exchange membrane materials: properties, modification and practical application]. Rossijskie Nano-tekhnologii — Russian Nanotechnologies, 4(3–4), 33–53 [in Russian]. DOI: https://doi.org/10.1134/S199507800903001X
Apel, P. Yu., & Dmitriev, S. N. (2004). Optimizaciya formy por trekovyh membrane [Optimization of the pore shape of track membranes]. Seriya. Kriticheskie Tekhnologii. Membrany — Series. Critical Technologies. Membranes, 3(23), 32–37 [in Rus-sian].
Berezina, N. P., Timofeev, S. V., Rolle, A. L., Fedorovich, N. V., & Dyuran-Vidal', S. (2002). Elektrotransportnye i strukturnye svojstva perftorirovannyh membran Nafion-117 i MF-4SK [Electrotransport and structural properties of perfluorinated membranes Nafion-117 and MF-4SC]. Elektrohimiya — Electrochemistry, 38(8), 1009–1015 [in Russian]. DOI: https://doi.org/10.1023/A:1016826131308
Karpenko, L. V., Demina, O. A., Dvorkina, G. A., Parshikov, S. B., Larshe, K., Okler, B., & Berezina, N. P. (2001). Sravnitel'noe izuchenie metodov opredeleniya udel'noj elektroprovodnosti ionoob-mennyh membrane [Comparative study of meth-ods for determining the specific conductivity of ion-exchange membranes]. Elektrohimiya — Electrochemistry, 37(3), 328–335 [in Russian]. DOI: https://doi.org/10.1023/A:1009081431563
Galama, A. H., Saakes, M., Bruning, H., Rijnaarts, H. H. M., & Post, J. W. (2014). Seawater predesalination with electrodi-alysis. Desalination, 342, 61–69. https://doi.org/10.1016/j.desal.2013.07.012 DOI: https://doi.org/10.1016/j.desal.2013.07.012
Gumirova, V. N., Razumovskaya, I. V., Apel, P. Yu., Bedin, S. A., & Bazhenov, S. L., Abdura-shidova, G. S. (2013). Meto-dy opredeleniya raspredeleniya por po poverhnosti trekovyh membrane [Methods for determining the distribution of pores on the surface of track membranes]. Prepodavatel' XXI Vek — Teacher XXI Century, 2 [in Russian].
Sanchez, C., Espinos, F. J., Barjola, A., Escorihuela, J., & Compañ, V. (2022). Hydrogen production from methanol–water solution and pure water electrolysis using nanocomposite perfluorinated sulfocationic membranes modified by polyaniline. Poly-mers, 14(21), 4500. https://doi.org/10.3390/polym14214500 DOI: https://doi.org/10.3390/polym14214500
Kravec, L. I., Yarmolenko, M. A., Rogachev, A. A., Gajnutdinov, R. V., Altynov, V. A., & Lizunov, N. E. (2021). Formiro-vanie na poverhnosti trekovyh membrangidrofobnyh pokrytij metodom el-ektronno-luchevogo dispergirovaniya polivinilhlorida v vakuume [Formation of hydrophobic mem-brane coatings on the surface of track films using the method of electron beam disper-sion of polyvinyl chloride in a vacuum]. Nanoindustriya Rossii — Nanoindustry of Russia, 14(6s), 44–54 [in Russian]. https://doi.org/10.22184/1993-8578.2021.14.6s.44.54 DOI: https://doi.org/10.22184/1993-8578.2021.14.6s.44.54
Djebara, M., Stoquert, J. P., Abdesselam, M., Muller, D., & Chami, A. C. (2012). FTIR analysis of polyethylene tereph-thalate irradiated by MeV He+. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 274, 70–77. https://doi.org/10.1016/j.nimb.2011.11.022 DOI: https://doi.org/10.1016/j.nimb.2011.11.022
Liang, Z., Chen, W., Liu, J., Wang, S., Zhou, Z., Li, W., Sun, G., & Xin, Q. (2004). FT-IR study of the microstructure of Nafion® membrane. Journal of Membrane Science, 233(1–2), 39–44. https://doi.org/10.1016/j.memsci.2003.12.008 DOI: https://doi.org/10.1016/j.memsci.2003.12.008
Kinumoto, T., Inaba, M., Nakayama, Y., Ogata, K., Umebayashi, R., Tasaka, A., Iriyama, Y., Abe, T., & Ogumi, Z. (2006). Durability of perfluorinated ionomer membrane against hydrogen peroxide. Journal of Power Sources, 158(2), 1222–1228. https://doi.org/10.1016/j.jpowsour.2005.10.043 DOI: https://doi.org/10.1016/j.jpowsour.2005.10.043
Déjardin, P., Vasina, E. N., Berezkin, V. V., Sobolev, V. D., & Volkov, V. I. (2005). Streaming potential in cylindrical pores of poly(ethylene terephthalate) track-etched membranes: variation of apparent ζ potential with pore radius. Langmuir, 21(10), 4680–4685. https://doi.org/10.1021/la046913e DOI: https://doi.org/10.1021/la046913e
Apel, P. Yu., Blonskaya, I. V., Ivanov, O. M., Kristavchuk, O. V., Lizunov, N. E., Nechaev, A. N., Orelovich, O. L., Polezhaeva, O. A., & Dmitriev, S. N. (2020). Creation of ion-selective membranes from polyethylene terephthalate films irradiated with heavy ions: critical parameters of the process. Membranes and Membrane Technologies, 2(2), 98–108. https://doi.org/10.1134/S251775162002002X DOI: https://doi.org/10.1134/S251775162002002X
Yaroslavtsev, A. B. (2012). Ion conductivity of composite materials on the base of solid electrolytes and ion-exchange membranes. Inorganic Materials, 48(13), 1193–1209. https://doi.org/10.1134/S0020168512130055 DOI: https://doi.org/10.1134/S0020168512130055
Ma, C.-H., Yu, T. L., Lin, H.-L., Huang, Y.-T., Chen, Y.-L., Jeng, U.-S., Lai, Y.-H., & Sun, Y.-S. (2009). Morphology and properties of Nafion membranes prepared by solution casting. Polymer, 50(7), 1764–1777. https://doi.org/10.1016/j.polymer.2009.01.060 DOI: https://doi.org/10.1016/j.polymer.2009.01.060
Safronova, E. Yu., Voropaeva, D. Yu., Safronov, D. V., Stretton, N., Parshina, A. V., & Yaroslavtsev, A. B. (2022). Correla-tion between Nafion morphology in various dispersion liquids and properties of the cast membranes. Membranes, 13(1), 13. https://doi.org/10.3390/membranes13010013 DOI: https://doi.org/10.3390/membranes13010013
Kim, R., Kim, H. G., Doo, G., Choi, C., Kim, S., Lee, J.-H., Heo, J., Jung, H.-Y., & Kim, H.-T. (2017). Ultrathin Nafion-filled porous membrane for zinc/bromine redox flow batteries. Scientific Reports, 7(1), 10503. https://doi.org/10.1038/s41598-017-10850-9 DOI: https://doi.org/10.1038/s41598-017-10850-9
Khandavalli, S., Park, J. H., Winter, H. H., Myers, D. J., Ulsh, M., & Mauger, S. A. (2023). Viscoelasticity enhancement and shear thickening of perfluorinated sulfonic acid ionomer dispersions in water–alcohol solvent mixtures. Macromolecules, 56(17), 6988–7005. https://doi.org/10.1021/acs.macromol.3c00383 DOI: https://doi.org/10.1021/acs.macromol.3c00383
Yamauchi, Y., Blonskaya, I. V., & Apel', P. Yu. (2019). Osmos v otricatel'no zaryazhennyh nanoka-pil-lyarah i ego usilenie anionnym poverhnosto-aktivnym veshchestvom [Osmosis in negatively charged nanocapillaries and its enhancement by cationic surfactant]. Kolloidnyj Zhurnal — Colloid Journal, 81(1), 125–136 [in Russian]. https://doi.org/10.1134/S0023291219010166 DOI: https://doi.org/10.1134/S0023291219010166
Larchet, C., Dammak, L., Auclair, B., Parchikov, S., & Nikonenko, V. (2004). A simplified procedure for ion-exchange membrane characterisation. New Journal of Chemistry, 28(10), 1260. https://doi.org/10.1039/b316725a DOI: https://doi.org/10.1039/b316725a
Kusoglu, A., & Weber, A. Z. (2017). New insights into perfluorinated sulfonic-acid ionomers. Chemical Reviews, 117(3), 987–1104. https://doi.org/10.1021/acs.chemrev.6b00159 DOI: https://doi.org/10.1021/acs.chemrev.6b00159
Yin, C., Wang, Z., Luo, Y., Li, J., Zhou, Y., Zhang, X., Zhang, H., Fang, P., & He, C. (2018). Thermal annealing on free volumes, crystallinity and proton conductivity of Nafion membranes. Journal of Physics and Chemistry of Solids, 120, 71–78. https://doi.org/10.1016/j.jpcs.2018.04.028 DOI: https://doi.org/10.1016/j.jpcs.2018.04.028
Kamel, M. S. A., Mohamed, H. F. M., Abdel-Hamed, M. O., & Abdel-Hady, E. E. (2019). Characterization and evaluation of Nafion HP JP as proton exchange membrane: Transport properties, nanostructure, morphology, and cell performance. Journal of Solid State Electrochemistry, 23(9), 2639–2656. https://doi.org/10.1007/s10008-019-04366-7 DOI: https://doi.org/10.1007/s10008-019-04366-7
Salmeron-Sanchez, I., Asenjo-Pascual, J., Avilés-Moreno, J. R., Pérez-Flores, J. C., Mauleón, P., & Ocón, P. (2022). Chem-ical physics insight of PPy-based modified ion exchange membranes: A fundamental approach. Journal of Membrane Science, 643, 120020. https://doi.org/10.1016/j.memsci.2021.120020 DOI: https://doi.org/10.1016/j.memsci.2021.120020
Falina, I. V., Demina, O. A., Kononenko, N. A., & Annikova, L. A. (2017). Influence of inert components on the formation of conducting channels in ion-exchange membranes. Journal of Solid State Electrochemistry, 21(3), 767–775. https://doi.org/10.1007/s10008-016-3415-0 DOI: https://doi.org/10.1007/s10008-016-3415-0
Luo, T., Abdu, S., & Wessling, M. (2018). Selectivity of ion exchange membranes: A review. Journal of Membrane Sci-ence, 555, 429–454. https://doi.org/10.1016/j.memsci.2018.03.051 DOI: https://doi.org/10.1016/j.memsci.2018.03.051
Kim, D.-H., Choi, Y.-E., Park, J.-S., & Kang, M.-S. (2019). Capacitive deionization employing pore-filled cation-exchange membranes for energy-efficient removal of multivalent cations. Electrochimica Acta, 295, 164–172. https://doi.org/10.1016/j.electacta.2018.10.124 DOI: https://doi.org/10.1016/j.electacta.2018.10.124
Kim, D.-H., & Kang, M.-S. (2018). Water electrolysis using pore-filled proton-exchange membranes for hydrogen water production. Chemistry Letters, 47(10), 1265–1268. https://doi.org/10.1246/cl.180560 DOI: https://doi.org/10.1246/cl.180560
Akter, M., & Park, J. -S. (2023). Fouling and mitigation behavior of foulants on ion exchange membranes with surface property in reverse electrodialysis. Membranes, 13(1), 106. https://doi.org/10.3390/membranes13010106 DOI: https://doi.org/10.3390/membranes13010106
Fan, H., Xu, Y., Zhao, F., Chen, Q. -B., Wang, D., & Wang, J. (2023). A novel porous asymmetric cation exchange mem-brane with thin selective layer for efficient electrodialysis desalination. Chemical Engineering Journal, 472, 144856. https://doi.org/10.1016/j.cej.2023.144856 DOI: https://doi.org/10.1016/j.cej.2023.144856
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Maria A. Ponomar, Veronika V. Sarapulova, Vera V. Gulyaeva , Pavel Yu. Apel , Natalia D. Pismenskaya

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



