Biological Compatibility of Polyethylene Terephthalate Track Membranes: Growth, Proliferation, and Viability of Cells in Culture Systems
DOI:
https://doi.org/10.31489/2959-0663/3-25-5Keywords:
track-etched membrane, poly(ethylene terephthalate), toxicity, tissue engineeringAbstract
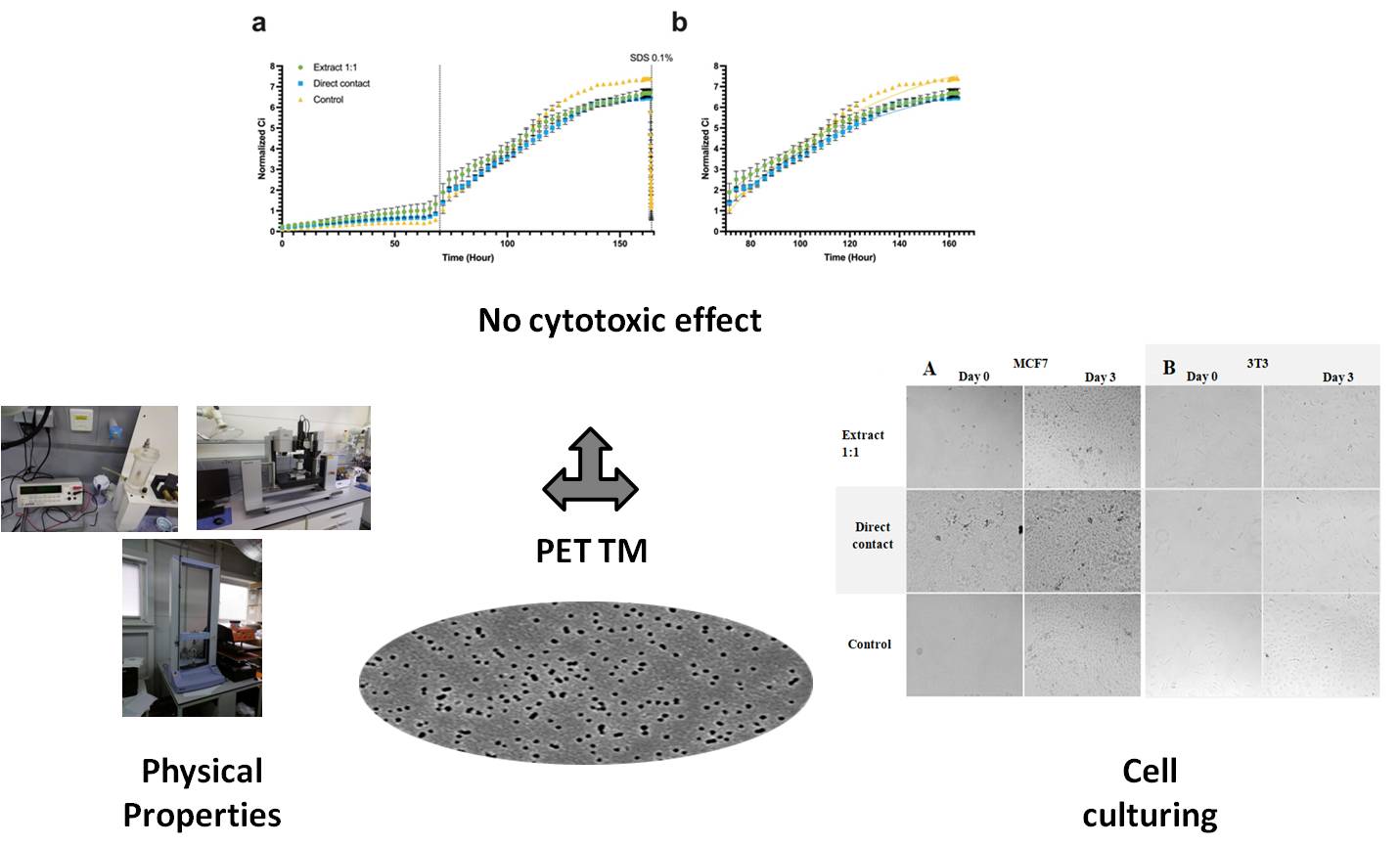
This study evaluated the biocompatibility of polyethylene terephthalate track membranes (PET TMs) obtained by heavy ion irradiation followed by chemical etching, with respect to cell growth, proliferation, and viability in culture systems. Physical parameters of the PET TMs were determined, including Young’s modulus, ultimate tensile strength, and contact angle. Cytotoxicity of PET TMs was studied on three cell cultures: the epithelial line MCF7 (adenocarcinoma), fibroblast-like mouse line 3T3, and a primary culture of mesenchymal stromal cells (MSCs) isolated from rabbit bone marrow. Cytotoxicity was assessed using two methods: extraction from the material and the direct contact method in accordance with the Interstate Standard ISO 10993-5-2011, IDT. The results demonstrated that neither PET TM extracts nor direct contact samples significantly affected cell growth. The proliferation rate of MCF7 cells was 0.01531 1/h for the extract and 0.01568 1/h for direct contact, which did not differ statistically from the control group (0.01877 1/h, p = 0.138). Microscopic analysis confirmed the preservation of cellular morphology: MCF7 cells retained cuboidal morphology, while 3T3 cells exhibited a spindle-shaped morphology. Real-time cell analysis (RTCA) revealed no significant effect of the tested samples on the cellular index (Ci), further supporting the absence of a cytotoxic effect. Visual observations of cell cultures after incubation with the studied samples also did not reveal cell confluence and morphology changes. These findings provide important evidence for the safety of PET TMs in biomedical research and cell culture systems, recommending them for further research in tissue engineering and regenerative medicine.
References
Apel, P. (2001). Track etching technique in membrane technology. Radiation Measurements, 34(5), 559–566. https://doi.org/10.1016/S1350-4487(01)00228-1 DOI: https://doi.org/10.1016/S1350-4487(01)00228-1
Dolfus, C., Piton, N., Toure, E., & Sabourin, J. C. (2015). Circulating tumor cell isolation: The assets of filtration methods with polycarbonate track-etched filters. Chinese Journal of Cancer Research, 27(5), 479–487. https://doi.org/10.3978/j.issn.1000-9604.2015.09.01
Kaya, D., & Keçeci, K. (2020). Review—Track-Etched Nanoporous Polymer Membranes as Sensors: A Review. Journal of The Electrochemical Society, 167(3), 037543. https://doi.org/10.1149/1945-7111/ab67a7 DOI: https://doi.org/10.1149/1945-7111/ab67a7
Mizuguchi, H., Sasaki, K., Ichinose, H., Seino, S., Sakurai, J., Iiyama, M., Kijima, T., Tachibana, K., Nishina, T., Taka-yanagi, T., & Shida, J. (2017). A triple-electrode based dual-biosensor system utilizing track-etched microporous membrane elec-trodes for the simultaneous determination of l-lactate and d-glucose. Bulletin of the Chemical Society of Japan, 90(11), 1211–1216. https://doi.org/10.1246/bcsj.20170193 DOI: https://doi.org/10.1246/bcsj.20170193
Torati, S. R., Reddy, V., Yoon, S. S., & Kim, C. (2016). Electrochemical biosensor for Mycobacterium tuberculosis DNA detection based on gold nanotubes array electrode platform. Biosensors and Bioelectronics, 78, 483–488. https://doi.org/10.1016/j.bios.2015.11.098 DOI: https://doi.org/10.1016/j.bios.2015.11.098
Barashkova, P. S., Molodkina, L. M., & Korovina, M. D. (2017). Both sided irradiated track membrane in local water sup-ply. Magazine of Civil Engineering, 71(3), 68–75. https://doi.org/10.18720/MCE.71.8
Russakova, A. V., Altynbaeva, L. Sh., Barsbay, M., Zheltov, D. A., Zdorovets, M. V., & Mashentseva, A. A. (2021). Kinetic and isotherm study of As(III) removal from aqueous solution by PET track-etched membranes loaded with copper microtubes. Membranes, 11(2), 116. https://doi.org/10.3390/membranes11020116 DOI: https://doi.org/10.3390/membranes11020116
Novo, P., Dell’Aica, M., Jender, M., Höving, S., Zahedi, R. P., & Janasek, D. (2017). Integration of polycarbonate mem-branes in microfluidic free-flow electrophoresis. Analyst, 142(22), 4228–4239. https://doi.org/10.1039/C7AN01514C DOI: https://doi.org/10.1039/C7AN01514C
Friedman, L. I., Hardwick, R. A., Daniels, J. R., Stromberg, R. R., & Ciarkowski, A. A. (1983). Evaluation of Membranes for Plasmapheresis. Artificial Organs, 7(4), 435–442. https://doi.org/10.1111/j.1525-1594.1983.tb04223.x DOI: https://doi.org/10.1111/j.1525-1594.1983.tb04223.x
Wright, C. W., Li, N., Shaffer, L., Fowler, S. C., Howell, K. L., Kumar, G., Nelson, E., Seddon, J. M., & George, M. (2023). Establishment of a 96-well transwell system using primary human gut organoids to capture multiple quantitative pathway readouts. Scientific Reports, 13, 16357. https://doi.org/10.1038/s41598-023-43656-z DOI: https://doi.org/10.1038/s41598-023-43656-z
Yamashita, T., Inui, T., Yokota, J., Kawakami, K., Morinaga, G., Takatani, M., Kishimoto, W., Tomita, J., & Mizuguchi, H. (2021). Monolayer platform using human biopsy-derived duodenal organoids for pharmaceutical research. Molecular Therapy — Methods & Clinical Development, 22, 263–278. https://doi.org/10.1016/j.omtm.2021.05.005 DOI: https://doi.org/10.1016/j.omtm.2021.05.005
George, J. H., Nagel, D., Waller, S., Hill, E. J., Parri, H. R., Coleman, M. D., Cui, Z., & Ye, H. (2018). A closer look at neu-ron interaction with track-etched microporous membranes. Scientific Reports, 8, Article 15552. https://doi.org/10.1038/s41598-018-33710-6 DOI: https://doi.org/10.1038/s41598-018-33710-6
Ippolitov, D., Arreza, L., Munir, M. N., & Hombach-Klonisch, S. (2022). Brain Microvascular Pericytes—More Than By-standers in Breast Cancer Brain Metastasis. In Cells, 11(8), 1263. https://doi.org/10.3390/cells11081263 DOI: https://doi.org/10.3390/cells11081263
Kumar, R., Harris-Hooker, S., Kumar, R., & Sanford, G. (2011). Co-culture of retinal and endothelial cells results in the modulation of genes critical to retinal neovascularization. Vascular Cell, 3, 27. https://doi.org/10.1186/2045-824X-3-27 DOI: https://doi.org/10.1186/2045-824X-3-27
Lu, Y., Ma, J., & Lin, G. (2019). Development of a two-layer transwell co-culture model for the in vitro investigation of pyrrolizidine alkaloid-induced hepatic sinusoidal damage. Food and Chemical Toxicology, 129, 391–398. https://doi.org/10.1016/j.fct.2019.04.057 DOI: https://doi.org/10.1016/j.fct.2019.04.057
Nishi, M., Tateishi, K., Sundararaj, J. S., Ino, Y., Nakai, Y., Hatayama, Y., Yamaoka, Y., Mihana, Y., Miyakawa, K., Ki-mura, H., Kimura, Y., Yamamoto, T., & Ryo, A. (2023). Development of a contacting transwell co-culture system for the in vitro propagation of primary central nervous system lymphoma. Frontiers in Cell and Developmental Biology, 11. https://doi.org/10.3389/fcell.2023.1275519 DOI: https://doi.org/10.3389/fcell.2023.1275519
Haynes, J., Palaniappan, B., Tsopmegha, E., & Sundaram, U. (2022). Regulation of nutrient and electrolyte absorption in human organoid-derived intestinal epithelial cell monolayers. Translational Research, 248, 22–35. https://doi.org/10.1016/j.trsl.2022.04.008 DOI: https://doi.org/10.1016/j.trsl.2022.04.008
Dokladny, K., In, J. G., Kaper, J., & Kovbasnjuk, O. (2021). Human epithelial stem cell-derived colonoid monolayers as a model to study shiga toxin-producing Escherichia coli–host interactions. Methods in Molecular Biology, 2291, 285–296. https://doi.org/10.1007/978-1-0716-1339-9_13 DOI: https://doi.org/10.1007/978-1-0716-1339-9_13
Kozuka, K., He, Y., Koo-McCoy, S., Kumaraswamy, P., Nie, B., Shaw, K., Chan, P., Leadbetter, M., He, L., Lewis, J. G., Zhong, Z., Charmot, D., Balaa, M., King, A. J., Caldwell, J. S., & Siegel, M. (2017). Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem cell reports, 9(6), 1976–1990. https://doi.org/10.1016/j.stemcr.2017.10.013 DOI: https://doi.org/10.1016/j.stemcr.2017.10.013
Bhatt, A. P., Gunasekara, D. B., Speer, J., Reed, M. I., Peña, A. N., Midkiff, B. R., Magness, S. T., Bultman, S. J., Allbritton, N. L., & Redinbo, M. R. (2018). Nonsteroidal Anti-Inflammatory Drug-Induced Leaky Gut Modeled Using Polarized Monolayers of Primary Human Intestinal Epithelial Cells. ACS infectious diseases, 4(1), 46–52. https://doi.org/10.1021/acsinfecdis.7b00139 DOI: https://doi.org/10.1021/acsinfecdis.7b00139
Lyck, R., Enzmann, G., Lécuyer, M. A., Abadier, M., Bradfield, P. F., Biechele, T., Wegner, A., Rüegg, S., Shimshek, D. R., Herich, L. C., Merz, P. A., Engelhardt, B., & Weksler, B. (2016). ALCAM (CD166) is involved in extravasation of monocytes ra-ther than T cells across the blood–brain barrier. Journal of Cerebral Blood Flow & Metabolism, 37(8), 2894–2909. https://doi.org/10.1177/0271678x16678639 DOI: https://doi.org/10.1177/0271678X16678639
Wimmer, I., Tietz, S., Nishihara, H., Deutsch, U., Sallusto, F., Gosselet, F., Lyck, R., Muller, W.A., Lassmann, H., Engel-hardt, B. (2019). PECAM-1 stabilizes blood-brain barrier integrity and favors paracellular T-cell diapedesis across the blood-brain barrier during neuroinflammation. Front. Immunol., 10, 711. https://doi.org/10.3389/fimmu.2019.00711 DOI: https://doi.org/10.3389/fimmu.2019.00711
Wilhelm, I., & Krizbai, I. A. (2014). In vitro models of the blood–brain barrier for the study of drug delivery to the brain. Molecular Pharmaceutics, 11(7), 1949–1963. https://doi.org/10.1021/mp500046f DOI: https://doi.org/10.1021/mp500046f
Wong, A. D., Ye, M., Levy, A. F., Rothstein, J. D., Bergles, D. E., & Searson, P. C. (2013). The blood–brain barrier: an en-gineering perspective. Frontiers in Neuroengineering, 6, 7. https://doi.org/10.3389/fneng.2013.00007 DOI: https://doi.org/10.3389/fneng.2013.00007
Li, H.-X., Sun, X.-Y., Yang, S.-M., Wang, Q., & Wang, Z.-Y. (2018). Peroxiredoxin 1 promoted tumor metastasis and angi-ogenesis in colorectal cancer. Pathology Research and Practice, 214(5), 655–660. https://doi.org/10.1016/j.prp.2018.03.026 DOI: https://doi.org/10.1016/j.prp.2018.03.026
Zhao, J., Ma, X., & Xu, H. (2022). miR-29b-3p inhibits 22Rv1 prostate cancer cell proliferation through the YWHAE/BCL-2 regulatory axis. Oncology Letters, 24(2), 289. https://doi.org/10.3892/ol.2022.13409 DOI: https://doi.org/10.3892/ol.2022.13409
Wei, Y., Wang, Y., Zang, A., Shang, Y., Song, Z., Wang, Z., Wang, Y., & Yang, H. (2018). Inducible T-cell co-stimulators regulate the proliferation and invasion of human hepatocellular carcinoma HepG2 cells. Biological research, 51(1), 2. https://doi.org/10.1186/s40659-017-0150-7 DOI: https://doi.org/10.1186/s40659-017-0150-7
Pang, J., Li, G., Qian, H., Wu, Y., & Chen, Y. (2022). Secretory type II cGMP-dependent protein kinase blocks activation of PDGFRβ via Ser254 in gastric cancer cells. Cell biology international, 46(5), 747–754. https://doi.org/10.1002/cbin.1176629 DOI: https://doi.org/10.1002/cbin.11766
Jeon, J. H., Kim, D. K., Shin, Y., Kim, H. Y., Song, B., Lee, E. Y., & Kim, J. K. (2016). Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Experimental & Molecular Medicine, 48, e277. https://doi.org/10.1038/emm.2016.129 DOI: https://doi.org/10.1038/emm.2016.129
Wasielewska, J. M., Chaves, J. C. S., Johnston, R. L., Milton, L. A., Hernández, D., Chen, L., Song, J., Lee, W., Leinenga, G., Nisbet, R. M., Pébay, A., Götz, J., White, A. R., & Oikari, L. E. (2022). A sporadic Alzheimer's blood-brain barrier model for developing ultrasound-mediated delivery of Aducanumab and anti-Tau antibodies. Theranostics, 12(16), 6826–6847. https://doi.org/10.7150/thno.72685 DOI: https://doi.org/10.7150/thno.72685
Varshavskaya, K. B., Petrushanko, I. Y., Mitkevich, V. A., Barykin, E.P., Makarov, A. A. (2024). Post-translational modifi-cations of beta-amyloid alter its transport in the blood–brain barrier in vitro model. Frontiers in Molecular Neuroscience, 17, 1362581. https://doi.org/10.3389/fnmol.2024.1362581 DOI: https://doi.org/10.3389/fnmol.2024.1362581
Guo, B. B., Bellingham, S. A., & Hill, A. F. (2016). Stimulating the release of exosomes increases the intercellular transfer of prions. Journal of Biological Chemistry, 291(10). https://doi.org/10.1074/jbc.M115.684258 DOI: https://doi.org/10.1074/jbc.M115.684258
Rodrigues, P.V., de Godoy, J.V.P., Bosque, B.P., Carvalho, H.F. & Castro-Fonseca, M. (2022). Transcellular propagation of fibrillar α-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent. Scientific Reports, 12, 4168. https://doi.org/10.1038/s41598-022-08076-5 DOI: https://doi.org/10.1038/s41598-022-08076-5
Hartmann, E. S., Schluessel, S., Köhler, M. I., Beck, F., Redeker, J. I., Summer, B., Schönitzer, V., Fottner, A., & Mayer-Wagner, S. (2020). Fibroblast-like cells change gene expression of bone remodelling markers in transwell cultures. European Jour-nal of Medical Research, 25(1), 52. https://doi.org/10.1186/s40001-020-00453-y DOI: https://doi.org/10.1186/s40001-020-00453-y
Juste-Lanas, Y., Díaz-Valdivia, N., Llorente, A., Ikemori, R. & Alcaraz, J. (2023). 3D collagen migration patterns reveal a SMAD3-dependent and TGF-β1-independent mechanism of recruitment for tumour-associated fibroblasts in lung adenocarcinoma. British Journal of Cancer, 128(6), 967–981. https://doi.org/10.1038/s41416-022-02093-x DOI: https://doi.org/10.1038/s41416-022-02093-x
Bhatia, S. N., & Ingber, D. E. (2014). Microfluidic organs-on-chips. Nature Biotechnology, 32(8), 760–772. https://doi.org/10.1038/nbt.2989 DOI: https://doi.org/10.1038/nbt.2989
Shimasaki, T., Yamamoto, S., Omura, R., & Takahiro Ochiya, T. (2021). Novel platform for regulation of extracellular ves-icles and metabolites secretion from cells using a multi-linkable horizontal co-culture plate. Micromachines, 12(11), 1431. https://doi.org/10.3390/mi12111431 DOI: https://doi.org/10.3390/mi12111431
Henry, O. Y. F., Villenave, R., Cronce, M. J., Leineweber, W. D., Benz, M. A., & Ingber, D. E. (2017). Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab on a Chip, 17(13), 2264–2271. https://doi.org/10.1039/c7lc00155j DOI: https://doi.org/10.1039/C7LC00155J
Srinivasan, B., Kolli, A. R., Esch, M. B., Abaci, H. E., Shuler, M. L., & Hickman, J. J. (2015). TEER measurement tech-niques for in vitro barrier model systems. Journal of laboratory automation, 20(2), 107–126. https://doi.org/10.1177/2211068214561025 DOI: https://doi.org/10.1177/2211068214561025
Ahmed Al-Tameemi, Z. K., Khanam, R., & Shetty, P. (2024). Bisphenol-A Leaching from Polycarbonate 5-Gallon Water Bottles in the UAE: A Comprehensive Study. Nepal Journal of Epidemiology, 14(1), 1301–1309. https://doi.org/10.3126/nje.v14i1.59934 DOI: https://doi.org/10.3126/nje.v14i1.59934
Le, H. H., Carlson, E. M., Chua, J. P., & Belcher, S. M. (2008). Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicology letters, 176(2), 149–156. https://doi.org/10.1016/j.toxlet.2007.11.001 DOI: https://doi.org/10.1016/j.toxlet.2007.11.001
Hoekstra, E. J., & Simoneau, C. (2013). Release of bisphenol A from polycarbonate: a review. Critical reviews in food sci-ence and nutrition, 53(4), 386–402. https://doi.org/10.1080/10408398.2010.536919 DOI: https://doi.org/10.1080/10408398.2010.536919
Wang, T., Han, J., Duan, X., Xiong, B., Cui, X. S., Kim, N. H., Liu, H. L., & Sun, S. C. (2016). The toxic effects and possi-ble mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget, 7(22), 32554–32565. https://doi.org/10.18632/oncotarget.8689 DOI: https://doi.org/10.18632/oncotarget.8689
Cimmino, I., Fiory, F., Perruolo, G., Miele, C., Beguinot, F., Formisano, P., & Oriente, F. (2020). Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. International journal of molecular sciences, 21(16), 5761. https://doi.org/10.3390/ijms21165761 DOI: https://doi.org/10.3390/ijms21165761
Li, T., Bian, B., Ji, R., Zhu, X., Wo, X., Song, Q., Li, Z., Wang, F., & Jia, Y. (2024). Polyethylene Terephthalate Micro-plastic Exposure Induced Reproductive Toxicity Through Oxidative Stress and p38 Signaling Pathway Activation in Male Mice. Toxics, 12(11), 779. https://doi.org/10.3390/toxics12110779 DOI: https://doi.org/10.3390/toxics12110779
Zhang, H., Zhang, S., Duan, Z., & Wang, L. (2022). Pulmonary toxicology assessment of polyethylene terephthalate nano-plastic particles in vitro. Environment international, 162, 107177. https://doi.org/10.1016/j.envint.2022.107177 DOI: https://doi.org/10.1016/j.envint.2022.107177
Apel, P., Bondarenko, M., Yamauchi, Y., & Yaroshchuk, A. (2021). Osmotic Pressure and Diffusion of Ions in Charged Na-nopores. Langmuir, 37(48), 14089–14095. https://doi.org/10.1021/acs.langmuir.1c02267 DOI: https://doi.org/10.1021/acs.langmuir.1c02267
International Organization for Standardization. (2011). ISO 10993-5:2011 IDT, medical devices. Evaluation of the biologi-cal effect of medical devices, Part 5, Cytotoxicity studies: in vitro methods.
Apel, P. Y., Velizarov, S., Volkov, A. V., Eliseeva, T. V., Nikonenko, V. V., Parshina, A. V., Yaroslavtsev, A. B. (2022). Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membranes and Membrane Technologies, 4(2), 69–92. https://doi.org/10.1134/S2517751622020032 DOI: https://doi.org/10.1134/S2517751622020032
Sabbatovskii, K. G., Vilenskii, A. I., Sobolev, V. D., Kochnev, Y. K., & Mchedlishvili, B. V. (2012). Electrosurface and structural properties of poly(ethylene terephthalate) track membranes. Colloid Journal, 74(3), 328–333. https://doi.org/10.1134/S1061933X12010139 DOI: https://doi.org/10.1134/S1061933X12010139
Apel, P. Y. (2013). Track-etching. Encyclopedia of membrane science and technology. Wiley. https://doi.org/10.1002/9781118522318.emst040 DOI: https://doi.org/10.1002/9781118522318.emst040
Lettmann, C., Mo Èckel, D., & Staude, E. (1999). Permeation and tangential flow streaming potential measurements for electrokinetic characterization of track-etched microfiltration membranes. Journal of Membrane Science, 159, 243–251. https://doi.org/10.1016/S0376-7388(99)00067-8 DOI: https://doi.org/10.1016/S0376-7388(99)00067-8
Déjardin, P., Vasina, E. N., Berezkin, V. V., Sobolev, V. D., & Volkov, V. I. (2005). Streaming potential in cylindrical pores of poly(ethylene terephthalate) track-etched membranes: Variation of apparent ζ potential with pore radius. Langmuir, 21(10), 4680–4685. https://doi.org/10.1021/la046913e DOI: https://doi.org/10.1021/la046913e
Chang, H. Y., Huang, C. C., Lin, K. Y., Kao, W. L., Liao, H. Y., You, Y. W., Lin, J. H., Kuo, Y. T., Kuo, D. Y., & Shyue, J. J. (2014). Effect of surface potential on NIH3T3 cell adhesion and proliferation. Journal of Physical Chemistry C, 118(26), 14464–14470. https://doi.org/10.1021/jp504662c DOI: https://doi.org/10.1021/jp504662c
Chang, H. Y., Kao, W. L., You, Y. W., Chu, Y. H., Chu, K. J., Chen, P. J., Wu, C. Y., Lee, Y. H., & Shyue, J. J. (2016). Ef-fect of surface potential on epithelial cell adhesion, proliferation and morphology. Colloids and Surfaces B: Biointerfaces, 141, 179–186. https://doi.org/10.1016/j.colsurfb.2016.01.049 DOI: https://doi.org/10.1016/j.colsurfb.2016.01.049
Tamada, Y., Ikada, Y. (1993). Cell adhesion to plasma-treated polymer surfaces. Polymer, 34(10), 2208–2212. DOI: https://doi.org/10.1016/0032-3861(93)90752-V
Qiu, L., Hughes-Brittain, N., Bastiaansen, C., Peijs, T., & Wang, W. (2016). Responses of Vascular Endothelial Cells to Photoembossed Topographies on Poly(Methyl Methacrylate) Films. Journal of Functional Biomaterials, 7(4), 33. https://doi.org/10.3390/jfb7040033 DOI: https://doi.org/10.3390/jfb7040033
Margel, S., Vogler, E. A., Firment, L., Watt, T., Haynie, S., & Sogah, D. Y. (1993). Peptide, protein, and cellular interac-tions with self‐assembled monolayer model surfaces. Journal of Biomedical Materials Research, 27(12), 1463–1476. Portico. https://doi.org/10.1002/jbm.820271202 DOI: https://doi.org/10.1002/jbm.820271202
Rahmati, M., Silva, E. A., Reseland, J. E., A. Heyward, C., & Haugen, H. J. (2020). Biological responses to physicochemi-cal properties of biomaterial surface. Chemical Society Reviews, 49(15), 5178–5224. https://doi.org/10.1039/d0cs00103a DOI: https://doi.org/10.1039/D0CS00103A
Yi, B., Shen, Y., Tang, H., Wang, X., & Zhang, Y. (2020). Stiffness of the aligned fibers affects structural and functional in-tegrity of the oriented endothelial cells. Acta biomaterialia, 108, 237–249. https://doi.org/10.1016/j.actbio.2020.03.022 DOI: https://doi.org/10.1016/j.actbio.2020.03.022
Yi, B., Shen, Y., Tang, H., Wang, X., Li, B., & Zhang, Y. (2019). Stiffness of Aligned Fibers Regulates the Phenotypic Ex-pression of Vascular Smooth Muscle Cells. ACS applied materials & interfaces, 11(7), 6867–6880. https://doi.org/10.1021/acsami.9b00293 DOI: https://doi.org/10.1021/acsami.9b00293
Ghosh, S., Laha, M., Mondal, S., Sengupta, S., & Kaplan, D. L. (2009). In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials, 30(33), 6530–6540. https://doi.org/10.1016/j.biomaterials.2009.08.019 DOI: https://doi.org/10.1016/j.biomaterials.2009.08.019
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Maksim V. Balyasin , Genrikh V. Serpionov, Mikhail E. Krasheninnikov, Alexander N. Nechaev, Alexey V. Lyundup

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



