Galvanic Replacement-Assisted Synthesis of Cu–Ag Composite Membrane Catalysts for Potassium Ferricyanide Reduction
DOI:
https://doi.org/10.31489/2959-0663/3-25-6Keywords:
composite, track-etched membranes, galvanic replacement, reduction, photocatalysts, poly(ethylene terephthalate), silver, copper, bimetallicAbstract
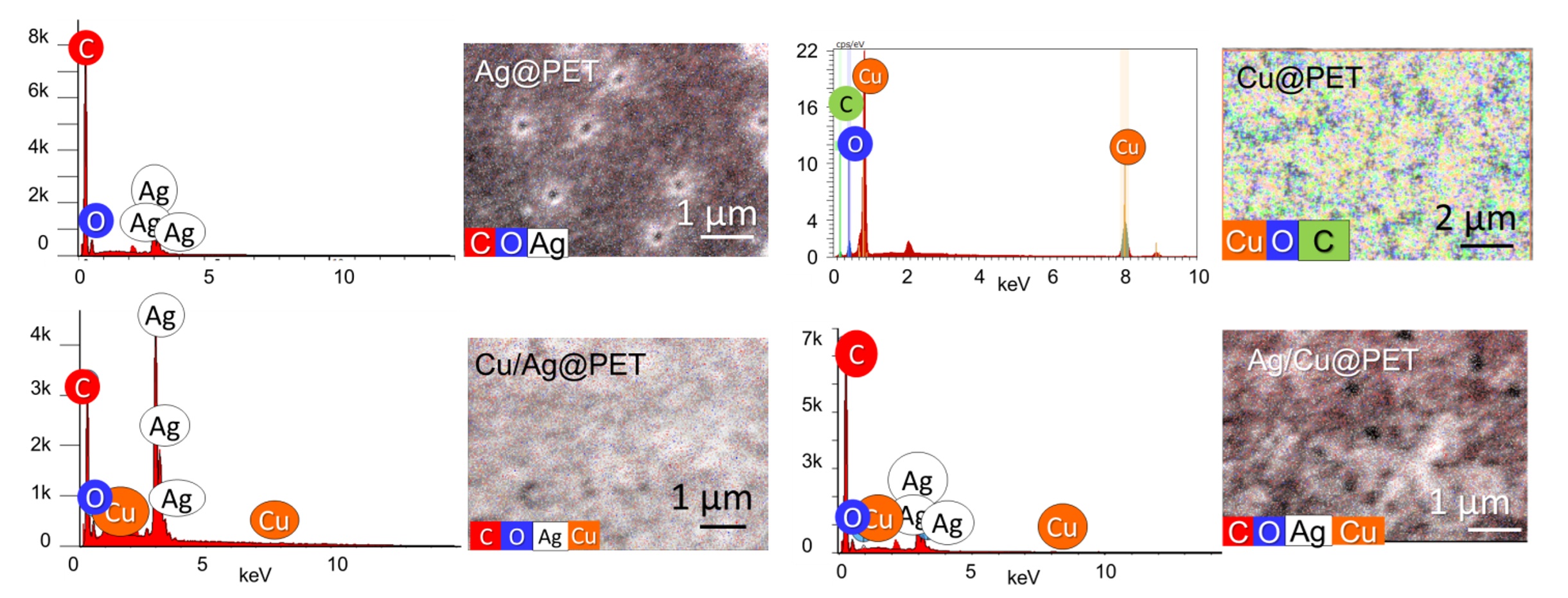
This study investigates the catalytic properties of mono- and bimetallic composite track-etched membranes (CTeMs) fabricated using a galvanic replacement strategy. Two bimetallic architectures, Ag/Cu@PET and Cu/Ag@PET, were synthesized by sequentially depositing copper and silver onto poly(ethylene terephthalate) (PET) templates. X-ray diffraction analysis revealed that doping Cu@PET with silver nanoparticles formed a substitutional solid solution (Ag₉₇Cu₃), which increased crystallinity by >45 % compared to monometallic Cu@PET. In contrast, doping Ag@PET with copper produced a two-layer tubular structure with phase-separated copper co-deposited along silver microtubes. The catalytic performance was evaluated through the pseudo-first-order reduction of potassium ferricyanide (PFC) by sodium borohydride. The Cu/Ag@PET composite with separate phases demonstrated superior activity, achieving 94.3 % PFC reduction within 40 minutes, significantly exceeding the performance of monometallic Ag@PET and Cu@PET. Kinetic analysis indicated that the rate constant and activation energy strongly depended on membrane structure and silver doping time in case of formation of substitutional solid solution phase. A minimum doping duration of 20 minutes was required for performance enhancement, with 30-minute Ag/Cu@PET samples reducing activation energy from 62.35 kJ/mol to 32.67 kJ/mol. These findings highlight the critical role of metal deposition order and structural configuration in optimizing catalytic activity, demonstrating the efficacy of galvanic replacement for designing high-performance, multi-metallic membrane catalysts.
References
Wellmann, P. J. (2021). The search for new materials and the role of novel processing routes. Discover Materials, 1(1), 14. https://doi.org/10.1007/s43939-021-00014-y DOI: https://doi.org/10.1007/s43939-021-00014-y
Magliaro, J., Altenhof, W., & Alpas, A. T. (2022). A review of advanced materials, structures and deformation modes for adaptive energy dissipation and structural crashworthiness. Thin-Walled Structures, 180, 109808. https://doi.org/10.1016/j.tws.2022.109808 DOI: https://doi.org/10.1016/j.tws.2022.109808
Kumari, S., Raturi, S., Kulshrestha, S., Chauhan, K., Dhingra, S., András, K., Singh, T. (2023). A comprehensive review on various techniques used for synthesizing nanoparticles. Journal of Materials Research and Technology, 27, 1739–1763. https://doi.org/10.1016/j.jmrt.2023.09.291 DOI: https://doi.org/10.1016/j.jmrt.2023.09.291
Baig, N., Kammakakam, I., & Falath, W. (2021). Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Materials Advances, 2(6), 1821–1871. https://doi.org/10.1039/D0MA00807A DOI: https://doi.org/10.1039/D0MA00807A
Lahiri, A., Pulletikurthi, G., & Endres, F. (2019). A Review on the Electroless Deposition of Functional Materials in Ionic Liquids for Batteries and Catalysis. Frontiers in Chemistry, 7, 85. https://doi.org/10.3389/fchem.2019.00085 DOI: https://doi.org/10.3389/fchem.2019.00085
Muench, F. (2023). Direct surface functionalization with metal and metal oxide nanostructures. In Encyclopedia of Nano-materials (pp. 318–336). Elsevier. https://doi.org/10.1016/B978-0-12-822425-0.00048-8 DOI: https://doi.org/10.1016/B978-0-12-822425-0.00048-8
Felix, E., Muench, F., & Ensinger, W. (2014). Green plating of high aspect ratio gold nanotubes and their morphology-dependent performance in enzyme-free peroxide sensing. RSC Adv., 4(47), 24504. https://doi.org/10.1039/c4ra03377a DOI: https://doi.org/10.1039/c4ra03377a
Schaefer, S., Muench, F., Mankel, E., Fuchs, A., Brötz, J., Kunz, U., & Ensinger, W. (2015). Double-Walled Ag — Pt Nano-tubes Fabricated by Galvanic Replacement and Dealloying: Effect of Composition on the Methanol Oxidation Activity. Nano, 10(06), 1550085. https://doi.org/10.1142/S179329201550085X DOI: https://doi.org/10.1142/S179329201550085X
Parmanbek, N., Aimanova, N. A., Mashentseva, A. A., Barsbay, M., Abuova, F. U., Nurpeisova, D. T., … Zdorovets, M. V. (2023). e-Beam and γ-rays Induced Synthesis and Catalytic Properties of Copper Nanoclusters-Deposited Composite Track-Etched Membranes. Membranes, 13(7), 659. https://doi.org/10.3390/membranes13070659 DOI: https://doi.org/10.3390/membranes13070659
Mashentseva, A. A., Kozlovskiy, A. L., Turapbay, K. O., Temir, A. M., Seytbaev, A. S., & Zdorovets, M. V. (2018). Deter-mination of Optimal Conditions for Electoless Synthesis of Copper Nanotubes in the Polymer Matrix. Russian Journal of General Chemistry, 88(6), 1213–1218. https://doi.org/10.1134/S1070363218060270 DOI: https://doi.org/10.1134/S1070363218060270
Muench, F., Rauber, M., Stegmann, C., Lauterbach, S., Kunz, U., Kleebe, H. -J., & Ensinger, W. (2011). Ligand-optimized electroless synthesis of silver nanotubes and their activity in the reduction of 4-nitrophenol. Nanotechnology, 22(41), 415602. https://doi.org/10.1088/0957-4484/22/41/415602 DOI: https://doi.org/10.1088/0957-4484/22/41/415602
Muench, F., Oezaslan, M., Svoboda, I., & Ensinger, W. (2015). Electroless plating of ultrathin palladium films: Self-initiated deposition and application in microreactor fabrication. Materials Research Express, 2(10), 105010. https://doi.org/10.1088/2053-1591/2/10/105010 DOI: https://doi.org/10.1088/2053-1591/2/10/105010
Mashentseva, A. A., Nurpeisova, D. T., & Barsbay, M. (2024). Effect of copper doping on the photocatalytic performance of Ni2O3@PC membrane composites in norfloxacin degradation. RSC Advances, 14(7), 4424–4435. https://doi.org/10.1039/d3ra07471d DOI: https://doi.org/10.1039/D3RA07471D
Nurpeisova, D. T., Mashentseva, A. A., Abuova, F., Aleskhanova, S. H., & Barsbay, M. (2025). Results in Materials Highly efficient CuO / Cu @ PC composite membranes for the photocatalytic degradation and sorption of roxithromycin from aqueous solutions. Results in Materials, 26(June 2025), 100677. https://doi.org/10.1016/j.rinma.2025.100677 DOI: https://doi.org/10.1016/j.rinma.2025.100677
Altynbaeva, L. S., Mashentseva, A. A., Aimanova, N. A., Zheltov, D. A., Shlimas, D. I., Nurpeisova, D. T., … Zdorovets, M. V. (2023). Eco-Friendly Electroless Template Synthesis of Cu-Based Composite Track-Etched Membranes for Sorption Removal of Lead(II) Ions. Membranes, 13(5), 495. https://doi.org/10.3390/membranes13050495 DOI: https://doi.org/10.3390/membranes13050495
Russakova, A. V., Altynbaeva, L. S., Barsbay, M., Zheltov, D. A., Zdorovets, M. V., & Mashentseva, A. A. (2021). Kinetic and Isotherm Study of As(III) Removal from Aqueous Solution by PET Track-Etched Membranes Loaded with Copper Microtubes. Membranes, 11(2), 116. https://doi.org/10.3390/membranes11020116 DOI: https://doi.org/10.3390/membranes11020116
Cheng, H., Wang, C., Qin, D., & Xia, Y. (2023). Galvanic Replacement Synthesis of Metal Nanostructures: Bridging the Gap between Chemical and Electrochemical Approaches. Accounts of Chemical Research, 56(7), 900–909. https://doi.org/10.1021/acs.accounts.3c00067 DOI: https://doi.org/10.1021/acs.accounts.3c00067
da Silva, A. G. M., Rodrigues, T. S., Haigh, S. J., & Camargo, P. H. C. (2017). Galvanic replacement reaction: recent devel-opments for engineering metal nanostructures towards catalytic applications. Chemical Communications, 53(53), 7135–7148. https://doi.org/10.1039/C7CC02352A DOI: https://doi.org/10.1039/C7CC02352A
Altynbaeva, L., Barsbay, M., Aimanova, N., Jakupova, Z., Nurpeisova, D., Zdorovets, M., & Mashentseva, A. (2022). A Novel Cu2O/ZnO@PET Composite Membrane for the Photocatalytic Degradation of Carbendazim. Nanomaterials, 12(10), 1724. https://doi.org/10.3390/nano12101724 DOI: https://doi.org/10.3390/nano12101724
Korolkov, I. V., Güven, O., Mashentseva, A. A., Atıcı, A. B., Gorin, Y. G., Zdorovets, M. V., & Taltenov, A. A. (2017). Ra-diation induced deposition of copper nanoparticles inside the nanochannels of poly(acrylic acid)-grafted poly(ethylene tereph-thalate) track-etched membranes. Radiation Physics and Chemistry, 130, 480–487. https://doi.org/10.1016/j.radphyschem.2016.10.006 DOI: https://doi.org/10.1016/j.radphyschem.2016.10.006
Korolkov, I. V., Mashentseva, A. A., Güven, O., Gorin, Y. G., Kozlovskiy, A. L., Zdorovets, M. V., … Cholach, S. O. (2018). Electron/gamma radiation-induced synthesis and catalytic activity of gold nanoparticles supported on track-etched poly(ethylene terephthalate) membranes. Materials Chemistry and Physics, 217(April), 31–39. https://doi.org/10.1016/j.matchemphys.2018.06.039 DOI: https://doi.org/10.1016/j.matchemphys.2018.06.039
Sut, M., Repmann, F., & Raab, T. (2014). Stability of Prussian Blue in Soils of a Former Manufactured Gas Plant Site. Soil and Sediment Contamination: An International Journal, 23(5), 504–522. https://doi.org/10.1080/15320383.2014.839626 DOI: https://doi.org/10.1080/15320383.2014.839626
Pearce, J. (1994). Studies of any toxicological effects of Prussian blue compounds in mammals—A review. Food and Chemical Toxicology, 32(6), 577–582. https://doi.org/10.1016/0278-6915(94)90116-3 DOI: https://doi.org/10.1016/0278-6915(94)90116-3
Hantson, P., N’Geye, P., Laforge, M., Clemessy, J. -L., & Baud, F. (1996). Suicide Attempt by Ingestion of Potassium Ferri-cyanide. Journal of Toxicology: Clinical Toxicology, 34(4), 471–473. https://doi.org/10.3109/15563659609013821 DOI: https://doi.org/10.3109/15563659609013821
Veerakumar, P., Salamalai, K., Thanasekaran, P., & Lin, K. C. (2018). Simple Preparation of Porous Carbon-Supported Ru-thenium: Propitious Catalytic Activity in the Reduction of Ferrocyanate(III) and a Cationic Dye. ACS Omega, 3(10), 12609–12621. research-article. https://doi.org/10.1021/acsomega.8b01680 DOI: https://doi.org/10.1021/acsomega.8b01680
Paolella, A., Faure, C., Timoshevskii, V., Marras, S., Bertoni, G., Guerfi, A., … Zaghib, K. (2017). A review on hexacy-anoferrate-based materials for energy storage and smart windows: challenges and perspectives. Journal of Materials Chemistry A, 5(36), 18919–18932. https://doi.org/10.1039/C7TA05121B DOI: https://doi.org/10.1039/C7TA05121B
Caballero, B., Toldra, P., & Fidel, F. (Eds.). (2003). Encyclopedia of Food Sciences and Nutrition (2nd-nd ed.). Academic Press Inc.
Huo, M., Zhao, R., Ying, Z., Jin, X., Zhu, Y., Wei, Q., & Ren, X. (2025). Efficient separation of Fe3+ and Cr3+ from chromi-um sludge leaching solution based on hydrogen bonding using trialkyl Phosphorus oxide. Separation and Purification Technology, 364, 132462. https://doi.org/10.1016/j.seppur.2025.132462 DOI: https://doi.org/10.1016/j.seppur.2025.132462
Touzi, N., & Horchani-Naifer, K. (2023). A study on the preparation and characterization of pigment quality from mill scale steel wastes. Environmental Science and Pollution Research, 31(28), 40538–40553. https://doi.org/10.1007/s11356-023-25594-5 DOI: https://doi.org/10.1007/s11356-023-25594-5
Berker, K. I., Güçlü, K., Tor, İ., & Apak, R. (2007). Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Ta-lanta, 72(3), 1157–1165. https://doi.org/10.1016/j.talanta.2007.01.019 DOI: https://doi.org/10.1016/j.talanta.2007.01.019
Cheong, Y. H., Ge, L., Zhao, N., Teh, L. K., & Lisak, G. (2020). Ion selective electrodes utilizing a ferrocyanide doped re-dox active screen-printed solid contact — impact of electrode response to conditioning. Journal of Electroanalytical Chemistry, 870, 114262. https://doi.org/10.1016/j.jelechem.2020.114262 DOI: https://doi.org/10.1016/j.jelechem.2020.114262
Altynbaeva, L. S., Mendibaeva, A. Z., Aimanova, N. A., Nurmakhan, A. E., Dzhakupova, Z. E., Tuleuov, B. I., & Mash-entseva, A. A. (2021). Kinetic and thermodynamic characteristics of the potassium hexationoferrate (III) decomposition catalytic reaction in the presence of composite track-etched membranes. NNC RK Bulletin, (1), 15–24. https://doi.org/10.52676/1729-7885-2021-1-15-24 DOI: https://doi.org/10.52676/1729-7885-2021-1-15-24
Mashentseva, A. A., Barsbay, M., Aimanova, N. A., & Zdorovets, M. V. (2021). Application of Silver-Loaded Composite Track-Etched Membranes for Photocatalytic Decomposition of Methylene Blue under Visible Light. Membranes, 11(1), 60. https://doi.org/10.3390/membranes11010060 DOI: https://doi.org/10.3390/membranes11010060
Mashentseva, A. A. (2019). Effect of the Oxidative Modification and Activation of Templates Based on Poly(ethylene ter-ephthalate) Track-Etched Membranes on the Electroless Deposition of Copper and the Catalytic Properties of Composite Mem-branes. Petroleum Chemistry, 59(12), 1337–1344. https://doi.org/10.1134/S0965544119120089 DOI: https://doi.org/10.1134/S0965544119120089
Borgekov, D., Mashentseva, A., Kislitsin, S., Kozlovskiy, A., Russakova, A., & Zdorovets, M. (2015). Temperature Depend-ent Catalytic Activity of Ag/PET Ion-Track Membranes Composites. Acta Physica Polonica A, 128(5), 871–875. https://doi.org/10.12693/APhysPolA.128.871 DOI: https://doi.org/10.12693/APhysPolA.128.871
Susana, C. R., Jorge, P. J., Pablo, H., Luis, M. L. M., & Paul, M. (2010). Colloidal gold-catalyzed reduction of ferrocyanate (III) by borohydride ions: A model system for redox catalysis. Langmuir, 26(2), 1271–1277. https://doi.org/10.1021/la902442p DOI: https://doi.org/10.1021/la902442p
Yen, C. W., & El-Sayed, M. A. (2009). Plasmonic field effect on the hexacyanoferrate (III)-thiosulfate electron transfer cat-alytic reaction on gold nanoparticles: Electromagnetic or thermal? Journal of Physical Chemistry C, 113(45), 19585–19590. https://doi.org/10.1021/jp905186g DOI: https://doi.org/10.1021/jp905186g
Martić, N., Reller, C., Macauley, C., Löffler, M., Reichert, A. M., Reichbauer, T., … Schmid, G. (2020). Ag2Cu2O3 — a catalyst template material for selective electroreduction of CO to C2+ products. Energy & Environmental Science, 13(9), 2993–3006. https://doi.org/10.1039/D0EE01100B DOI: https://doi.org/10.1039/D0EE01100B
Wang, L., Higgins, D. C., Ji, Y., Morales-Guio, C. G., Chan, K., Hahn, C., & Jaramillo, T. F. (2020). Selective reduction of CO to acetaldehyde with CuAg electrocatalysts. Proceedings of the National Academy of Sciences, 117(23), 12572–12575. https://doi.org/10.1073/pnas.1821683117 DOI: https://doi.org/10.1073/pnas.1821683117
Rollier, F. A., Muravev, V., Kosinov, N., Wissink, T., Anastasiadou, D., Ligt, B., … Hensen, E. J. M. (2025). Cu–Ag inter-actions in bimetallic Cu–Ag catalysts enhance C 2+ product formation during electrochemical CO reduction. Journal of Materials Chemistry A, 13(3), 2285–2300. https://doi.org/10.1039/D4TA04263H DOI: https://doi.org/10.1039/D4TA04263H
Kim, Y., Dumett Torres, D., & Jain, P. K. (2016). Activation Energies of Plasmonic Catalysts. Nano Letters, 16(5), 3399–3407. https://doi.org/10.1021/acs.nanolett.6b01373 DOI: https://doi.org/10.1021/acs.nanolett.6b01373
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Rakisheva Saniya, Dinara T. Nurpeisova, Alisher M. Zhumabayev, Nursanat Parmanbek , Murat Barsbay, Anastassiya A. Mashentseva

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC-ND 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.



